Risperidone - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for risperidone and what is the scope of freedom to operate?
Risperidone
is the generic ingredient in seven branded drugs marketed by Labs Farms Rovi Sa, Shandong Luye, Indivior, Janssen Pharms, Teva Pharms Usa Inc, Amneal Pharms, Ani Pharms, Aurobindo Pharma Ltd, Chartwell Molecular, Hikma, Lannett Co Inc, Pharm Assoc, Precision Dose, Sciegen Pharms Inc, Taro, Tris Pharma Inc, Wockhardt, Teva, Actavis Labs Fl Inc, Chartwell Rx, Dash Pharms, Dr Reddys Labs Ltd, Endo Operations, Jubilant Generics, Sandoz, Sun Pharm Inds Ltd, Zydus Pharms Usa, Ajanta Pharma Ltd, Amneal, Apotex Inc, Esjay Pharma, Heritage Pharma Avet, Ipca Labs Ltd, Jubilant Cadista, Prinston Inc, Ratiopharm, Renata, Rising, Sun Pharm Inds Inc, Synthon Pharms, Torrent Pharms, Watson Labs, West Ward Pharms, and Zydus Pharms Usa Inc, and is included in fifty-four NDAs. There are thirty-eight patents protecting this compound and two Paragraph IV challenges. Additional information is available in the individual branded drug profile pages.Risperidone has three hundred and thirty-two patent family members in forty-three countries.
There are thirty drug master file entries for risperidone. Thirty-nine suppliers are listed for this compound. There is one tentative approval for this compound.
Summary for risperidone
| International Patents: | 332 |
| US Patents: | 38 |
| Tradenames: | 7 |
| Applicants: | 44 |
| NDAs: | 54 |
| Drug Master File Entries: | 30 |
| Finished Product Suppliers / Packagers: | 39 |
| Raw Ingredient (Bulk) Api Vendors: | 143 |
| Clinical Trials: | 557 |
| Patent Applications: | 7,021 |
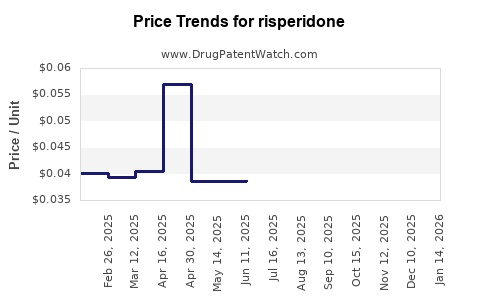
| Drug Prices: | Drug price trends for risperidone |
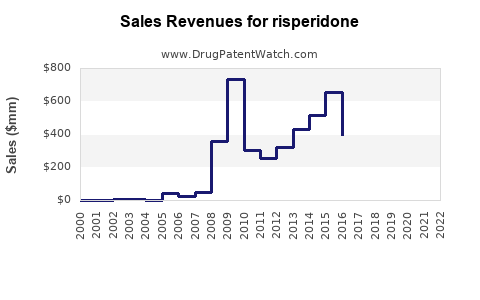
| Drug Sales Revenues: | Drug sales revenues for risperidone |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for risperidone |
| What excipients (inactive ingredients) are in risperidone? | risperidone excipients list |
| DailyMed Link: | risperidone at DailyMed |
Recent Clinical Trials for risperidone
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Assiut University | Phase 4 |
| Consorcio Centro de Investigación Biomédica en Red (CIBER) | Phase 4 |
| Instituto de Salud Carlos III | Phase 4 |
Generic filers with tentative approvals for RISPERIDONE
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
Pharmacology for risperidone
| Drug Class | Atypical Antipsychotic |
Medical Subject Heading (MeSH) Categories for risperidone
Anatomical Therapeutic Chemical (ATC) Classes for risperidone
Paragraph IV (Patent) Challenges for RISPERIDONE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| RISPERDAL | Orally Disintegrating Tablets | risperidone | 0.25 mg | 021444 | 1 | 2005-04-11 |
| RISPERDAL | Orally Disintegrating Tablets | risperidone | 3 mg and 4 mg | 021444 | 1 | 2005-03-23 |
US Patents and Regulatory Information for risperidone
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| West Ward Pharms | RISPERIDONE | risperidone | TABLET;ORAL | 078740-001 | May 29, 2009 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Synthon Pharms | RISPERIDONE | risperidone | TABLET;ORAL | 078187-004 | Oct 22, 2009 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Teva | UZEDY | risperidone | SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 213586-005 | Apr 28, 2023 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Indivior | PERSERIS KIT | risperidone | FOR SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 210655-001 | Jul 27, 2018 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| West Ward Pharms | RISPERIDONE | risperidone | TABLET;ORAL | 078740-004 | May 29, 2009 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Teva | UZEDY | risperidone | SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 213586-001 | Apr 28, 2023 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Ipca Labs Ltd | RISPERIDONE | risperidone | TABLET;ORAL | 205104-006 | Jun 26, 2024 | AB | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for risperidone
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Janssen Pharms | RISPERDAL CONSTA | risperidone | INJECTABLE;INTRAMUSCULAR | 021346-001 | Oct 29, 2003 | ⤷ Sign Up | ⤷ Sign Up |
| Janssen Pharms | RISPERDAL CONSTA | risperidone | INJECTABLE;INTRAMUSCULAR | 021346-001 | Oct 29, 2003 | ⤷ Sign Up | ⤷ Sign Up |
| Janssen Pharms | RISPERDAL CONSTA | risperidone | INJECTABLE;INTRAMUSCULAR | 021346-003 | Oct 29, 2003 | ⤷ Sign Up | ⤷ Sign Up |
| Janssen Pharms | RISPERDAL | risperidone | SOLUTION;ORAL | 020588-001 | Jun 10, 1996 | ⤷ Sign Up | ⤷ Sign Up |
| Janssen Pharms | RISPERDAL CONSTA | risperidone | INJECTABLE;INTRAMUSCULAR | 021346-004 | Apr 12, 2007 | ⤷ Sign Up | ⤷ Sign Up |
| Janssen Pharms | RISPERDAL | risperidone | TABLET;ORAL | 020272-008 | May 10, 1999 | ⤷ Sign Up | ⤷ Sign Up |
| Janssen Pharms | RISPERDAL CONSTA | risperidone | INJECTABLE;INTRAMUSCULAR | 021346-001 | Oct 29, 2003 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for risperidone
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Laboratorios Farmacéuticos Rovi, S.A. | Okedi | risperidone | EMEA/H/C/005406 Treatment of schizophrenia in adults for whom tolerability and effectiveness has been established with oral risperidone. |
Authorised | no | no | no | 2022-02-14 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for risperidone
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 5882993 | ⤷ Sign Up | |
| Chile | 2012003350 | Composicion farmaceutica inyectable de liberacion controlada que comprende risperidona, un copolimero de acido lactico y glicolico, un disolvente miscible en agua, una viscosidad y una relacion de masas de disolvente/farmaco y de farmaco/polimero especificas; kit farmaceutico; y uso para tratar esquizofrenia o trastornos bipolares. | ⤷ Sign Up |
| European Patent Office | 2575890 | COMPOSITION RETARD INJECTABLE ANTIPSYCHOTIQUE (ANTIPSYCHOTIC INJECTABLE DEPOT COMPOSITION) | ⤷ Sign Up |
| Eurasian Patent Organization | 201390783 | БИОРАЗЛАГАЕМЫЕ КОМПОЗИЦИИ ДЛЯ ДОСТАВКИ ЛЕКАРСТВЕННЫХ СРЕДСТВ | ⤷ Sign Up |
| South Africa | 201501426 | INJECTABLE COMPOSITIONS COMPRISING LETROZOLE OR ANASTROZOLE | ⤷ Sign Up |
| South Korea | 101539313 | ⤷ Sign Up | |
| Slovenia | 2879661 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for risperidone
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0196132 | 94C0008 | Belgium | ⤷ Sign Up | PRODUCT NAME: RISPERIDONE; NAT REG.: 2 S 414 F 3 19940527; FIRST REG.: GB 0242/0186 19921208 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.