DAPAGLIFLOZIN - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for dapagliflozin and what is the scope of freedom to operate?
Dapagliflozin
is the generic ingredient in four branded drugs marketed by Astrazeneca Ab and is included in four NDAs. There are twenty patents protecting this compound. Additional information is available in the individual branded drug profile pages.Dapagliflozin has four hundred and forty patent family members in fifty-one countries.
There are twenty-six drug master file entries for dapagliflozin. Five suppliers are listed for this compound. There are twelve tentative approvals for this compound.
Summary for DAPAGLIFLOZIN
| International Patents: | 440 |
| US Patents: | 20 |
| Tradenames: | 4 |
| Applicants: | 1 |
| NDAs: | 4 |
| Drug Master File Entries: | 26 |
| Finished Product Suppliers / Packagers: | 5 |
| Raw Ingredient (Bulk) Api Vendors: | 109 |
| Clinical Trials: | 427 |
| Patent Applications: | 3,227 |
| Formulation / Manufacturing: | see details |
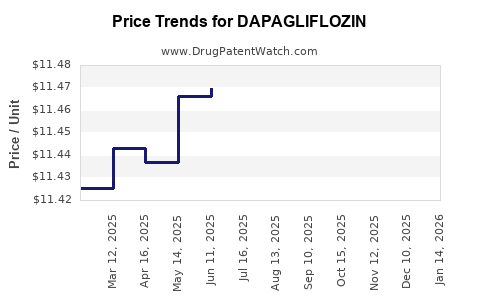
| Drug Prices: | Drug price trends for DAPAGLIFLOZIN |
| What excipients (inactive ingredients) are in DAPAGLIFLOZIN? | DAPAGLIFLOZIN excipients list |
| DailyMed Link: | DAPAGLIFLOZIN at DailyMed |
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for DAPAGLIFLOZIN
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for DAPAGLIFLOZIN
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Yale University | Phase 1 |
| Yale University | Phase 2 |
| American Heart Association | Phase 2 |
Generic filers with tentative approvals for DAPAGLIFLOZIN
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
Pharmacology for DAPAGLIFLOZIN
| Drug Class | Sodium-Glucose Cotransporter 2 Inhibitor |
| Mechanism of Action | Sodium-Glucose Transporter 2 Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for DAPAGLIFLOZIN
Paragraph IV (Patent) Challenges for DAPAGLIFLOZIN
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| FARXIGA | Tablets | dapagliflozin | 5 mg and 10 mg | 202293 | 20 | 2018-01-08 |
US Patents and Regulatory Information for DAPAGLIFLOZIN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astrazeneca Ab | XIGDUO XR | dapagliflozin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 205649-001 | Oct 29, 2014 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Astrazeneca Ab | QTERN | dapagliflozin; saxagliptin hydrochloride | TABLET;ORAL | 209091-002 | May 2, 2019 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Astrazeneca Ab | QTERNMET XR | dapagliflozin; metformin hydrochloride; saxagliptin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 210874-001 | May 2, 2019 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Astrazeneca Ab | QTERNMET XR | dapagliflozin; metformin hydrochloride; saxagliptin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 210874-002 | May 2, 2019 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Astrazeneca Ab | XIGDUO XR | dapagliflozin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 205649-002 | Oct 29, 2014 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for DAPAGLIFLOZIN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Astrazeneca Ab | FARXIGA | dapagliflozin | TABLET;ORAL | 202293-001 | Jan 8, 2014 | ⤷ Sign Up | ⤷ Sign Up |
| Astrazeneca Ab | FARXIGA | dapagliflozin | TABLET;ORAL | 202293-001 | Jan 8, 2014 | ⤷ Sign Up | ⤷ Sign Up |
| Astrazeneca Ab | FARXIGA | dapagliflozin | TABLET;ORAL | 202293-001 | Jan 8, 2014 | ⤷ Sign Up | ⤷ Sign Up |
| Astrazeneca Ab | FARXIGA | dapagliflozin | TABLET;ORAL | 202293-002 | Jan 8, 2014 | ⤷ Sign Up | ⤷ Sign Up |
| Astrazeneca Ab | FARXIGA | dapagliflozin | TABLET;ORAL | 202293-001 | Jan 8, 2014 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for DAPAGLIFLOZIN
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| AstraZeneca AB | Forxiga | dapagliflozin | EMEA/H/C/002322 Type 2 diabetes mellitusForxiga is indicated in adults and children aged 10 years and above for the treatment of insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exerciseas monotherapy when metformin is considered inappropriate due to intolerance.in addition to other medicinal products for the treatment of type 2 diabetes.For study results with respect to combination of therapies, effects on glycaemic control, cardiovascular and renal events, and the populations studied, see sections 4.4, 4.5 and 5.1.Heart failureForxiga is indicated in adults for the treatment of symptomatic chronic heart failure.Chronic kidney diseaseForxiga is indicated in adults for the treatment of chronic kidney disease. |
Authorised | no | no | no | 2012-11-11 | |
| AstraZeneca AB | Edistride | dapagliflozin | EMEA/H/C/004161 Type 2 diabetes mellitusEdistride is indicated in adults and children aged 10 years and above for the treatment of insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exerciseas monotherapy when metformin is considered inappropriate due to intolerance.in addition to other medicinal products for the treatment of type 2 diabetes.For study results with respect to combination of therapies, effects on glycaemic control, cardiovascular and renal events, and the populations studied, see sections 4.4, 4.5 and 5.1.Heart failureEdistride is indicated in adults for the treatment of symptomatic chronic heart failure.Chronic kidney diseaseEdistride is indicated in adults for the treatment of chronic kidney disease. |
Authorised | no | no | no | 2015-11-09 | |
| Viatris Limited | Dapagliflozin Viatris | dapagliflozin | EMEA/H/C/006006 Type 2 diabetes mellitusDapagliflozin Viatris is indicated in adults and children aged 10 years and above for the treatment of insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise- as monotherapy when metformin is considered inappropriate due to intolerance.- in addition to other medicinal products for the treatment of type 2 diabetes.For study results with respect to combination of therapies, effects on glycaemic control, cardiovascular and renal events, and the populations studied, see sections 4.4, 4.5 and 5.1.Heart failureDapagliflozin Viatris is indicated in adults for the treatment of symptomatic chronic heart failure with reduced ejection fraction.Chronic kidney diseaseDapagliflozin Viatris is indicated in adults for the treatment of chronic kidney disease. |
Authorised | yes | no | no | 2023-03-24 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for DAPAGLIFLOZIN
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| World Intellectual Property Organization (WIPO) | 2009153132 | ⤷ Sign Up | |
| Denmark | 1971362 | ⤷ Sign Up | |
| Russian Federation | 2002109477 | С-арилглюкозидные ингибиторы SGTL2 | ⤷ Sign Up |
| South Korea | 101059279 | ⤷ Sign Up | |
| Hong Kong | 1205689 | 乳酸-羥基乙酸 共聚物為基礎、含有多肽和糖的持續釋放微膠囊 (POLY(LACTIDE-CO-GLYCOLIDE)-BASED SUSTAINED RELEASE MICROCAPSULES COMPRISING A POLYPEPTIDE AND A SUGAR. (-)) | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for DAPAGLIFLOZIN
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2498758 | 122020000018 | Germany | ⤷ Sign Up | PRODUCT NAME: METFORMIN ODER EIN PHARMAZEUTISCH AKZEPTABLES SALZ DAVON; SAXAGLIPTIN ODER EIN PHARMAZEUTISCH AKZEPTABLES SALZ DAVON; DAPAGLIFLOZIN ODER EIN PHARMAZEUTISCH AKZEPTABLES SOLVAT DAVON; REGISTRATION NO/DATE: EU/1/19/1401 20191111 |
| 1506211 | 13C0022 | France | ⤷ Sign Up | PRODUCT NAME: DAPAGLIFLOZINE ET SES SELS PHARMACEUTIQUEMENT ACCEPTABLES; REGISTRATION NO/DATE: EU/1/12/795/001 20121112 |
| 2139494 | 301054 | Netherlands | ⤷ Sign Up | PRODUCT NAME: SAXAGLIPTIN AND DAPAGLIFLOZIN; REGISTRATION NO/DATE: EU/1/16/1108 20160719 |
| 1506211 | 1390017-0 | Sweden | ⤷ Sign Up | PRODUCT NAME: DAPAGLIFLOZIN OCH FARMACEUTISKT GODTAGBARA SALTER DAERAV; REG. NO/DATE: EU/1/12/795/001 20121112 |
| 1506211 | 300677 | Netherlands | ⤷ Sign Up | PRODUCT NAME: COMBINATIE VAN DAPAGLIFLOZINE OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN EN METFORMINE OF EEN FARMACEITISCH AANVAARDBAAR ZOUT DAARVAN, ZOALS BESCHERMD DOOR HET BASISOCTROOI EP 1506211B1; REGISTRATION NO/DATE: EU/1/13/900 20140121 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.