BENZOYL PEROXIDE - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for benzoyl peroxide and what is the scope of patent protection?
Benzoyl peroxide
is the generic ingredient in ten branded drugs marketed by Galderma Labs Lp, Bausch, Actavis Labs Ut Inc, Chartwell Rx, Encube, Glenmark Pharms, Mylan Pharms Inc, Padagis Israel, Taro, Zydus Pharms, Stiefel, Biofrontera, Valeant Intl, and Lyne, and is included in twenty-four NDAs. There are twenty-two patents protecting this compound. Additional information is available in the individual branded drug profile pages.Benzoyl peroxide has twenty-eight patent family members in eleven countries.
There are seven drug master file entries for benzoyl peroxide. One supplier is listed for this compound. There are two tentative approvals for this compound.
Summary for BENZOYL PEROXIDE
| International Patents: | 28 |
| US Patents: | 22 |
| Tradenames: | 10 |
| Applicants: | 14 |
| NDAs: | 24 |
| Drug Master File Entries: | 7 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 113 |
| Clinical Trials: | 107 |
| Patent Applications: | 5,591 |
| Formulation / Manufacturing: | see details |
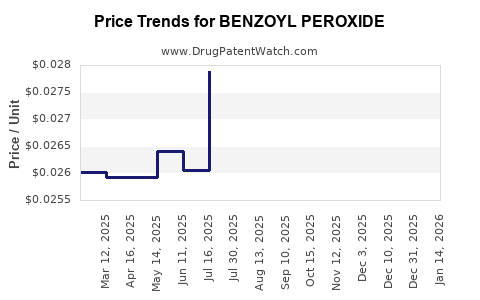
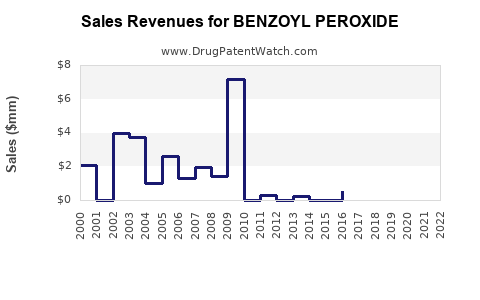
| Drug Prices: | Drug price trends for BENZOYL PEROXIDE |
| What excipients (inactive ingredients) are in BENZOYL PEROXIDE? | BENZOYL PEROXIDE excipients list |
| DailyMed Link: | BENZOYL PEROXIDE at DailyMed |
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for BENZOYL PEROXIDE
Generic Entry Date for BENZOYL PEROXIDE*:
Constraining patent/regulatory exclusivity:
Dosage:
CREAM;TOPICAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for BENZOYL PEROXIDE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| South Valley University | N/A |
| University of Missouri-Columbia | Phase 4 |
| University of Oklahoma | Phase 3 |
Generic filers with tentative approvals for BENZOYL PEROXIDE
| Applicant | Application No. | Strength | Dosage Form |
| ⤷ Try a Trial | ⤷ Try a Trial | 3.75%; EQ 1.2% BASE | GEL;TOPICAL |
| ⤷ Try a Trial | ⤷ Try a Trial | UNKNOWN | UNKNOWN |
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
US Patents and Regulatory Information for BENZOYL PEROXIDE
International Patents for BENZOYL PEROXIDE
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 5164840 | ⤷ Try a Trial | |
| China | 101277757 | Metal oxide coating of water insoluble ingredients | ⤷ Try a Trial |
| Israel | 189159 | חיפוי של מתכת אוקסיד למרכיבים בלתי מסיסים במים (Metal oxide coating of water insoluble ingredients) | ⤷ Try a Trial |
| South Korea | 20080039927 | METAL OXIDE COATING OF WATER INSOLUBLE INGREDIENTS | ⤷ Try a Trial |
| European Patent Office | 2431088 | Revêtement d'oxyde métallique d'ingrédients insolubles dans l'eau (Metal oxide coating of water insoluble ingredients) | ⤷ Try a Trial |
| Canada | 3130441 | METHODE DE TRAITEMENT DE SYMPTOMES D'ERYTHEME MODERE A SEVERE CHEZ DES PATIENTS ATTEINTS DE ROSACEE (METHOD FOR TREATMENT OF MODERATE TO SEVERE ERYTHEMA SYMPTOMS IN ROSACEA PATIENTS) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for BENZOYL PEROXIDE
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0186118 | SPC/GB05/029 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: MESOTRIONE (2-(4-METHYLSULPHONYL-2-NITROBENZOYL)-1,3CYCLOHEXANEDIONE); REGISTERED: AU 2726 20001016; UK 0309 OF 2005 20050218 |
| 1667986 | 92172 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: SOLVAT ACETONIQUE DU CABAZITAXEL, OU DESIGNE SOLVAT ACETONIQUE DU DIMETHOXY DOCETAXEL OU SOLVAT ACETONIQUE DU (2R,3S)-3-TERT-BUTOXYCARBONYLAMINO-2-HYDROXY-3-PHENYLPROPIONATE DE 4-ACETOXY-2A-BENZOYLOXY-5BETA,20-EPOXY-1-HYDROXY-7BETA,10A-DIMETHOXY-9-OXO-TAX-11-ENE-13A-YLE(ACETONATE DU CABAZITAXEL) |
| 1586316 | SPC/GB11/054 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: BROMFENAC 2-AMINO-3-(4-BROMOBENZOYL)PHENYLACETIC ACID OR A PHARMACOLOGICALLY ACCEPTABLE SALT THEREOF OR A HYDRATE THEREOF; REGISTERED: UK EU/1/11/692/001 20110523 |
| 1458369 | 122008000041 | Germany | ⤷ Try a Trial | PRODUCT NAME: ADAPALEN IN KOMBINATION MIT BENZOYLPEROXID; NAT. REGISTRATION NO/DATE: 67913.00.00 20080229; FIRST REGISTRATION: DAENEMARK 40440 20071218 |
| 0591275 | SPC/GB05/030 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: NITISINONE (2-(2-NITRO-4-TRIFLUOROMETHYLBENZOYL)-1,3-CYCLOHEXANEDIONE) OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REGISTERED: UK EU/1/04/303/001 20050221; UK EU/1/04/303/002 20050221; UK EU/1/04/303/003 20050221 |
| 1458369 | SPC/GB10/005 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: ADAPALENE AND BENZOYL PEROXIDE; REGISTERED: DK 40440 20071218; UK PL10590/0057 20091111 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |