Teva Branded Pharm Company Profile
✉ Email this page to a colleague
What is the competitive landscape for TEVA BRANDED PHARM, and what generic alternatives to TEVA BRANDED PHARM drugs are available?
TEVA BRANDED PHARM has thirty-four approved drugs.
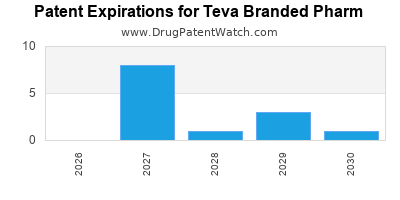
There are fifty-nine US patents protecting TEVA BRANDED PHARM drugs.
There are six hundred and eighty-six patent family members on TEVA BRANDED PHARM drugs in forty-one countries and thirty-nine supplementary protection certificates in fourteen countries.
Summary for Teva Branded Pharm
| International Patents: | 686 |
| US Patents: | 59 |
| Tradenames: | 34 |
| Ingredients: | 20 |
| NDAs: | 34 |
| Patent Litigation for Teva Branded Pharm: | See patent lawsuits for Teva Branded Pharm |
Drugs and US Patents for Teva Branded Pharm
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Teva Branded Pharm | PROAIR DIGIHALER | albuterol sulfate | POWDER, METERED;INHALATION | 205636-002 | Dec 21, 2018 | RX | Yes | No | 8,651,103 | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Teva Branded Pharm | AUSTEDO | deutetrabenazine | TABLET;ORAL | 208082-003 | Apr 3, 2017 | RX | Yes | Yes | 10,959,996*PED | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Teva Branded Pharm | NORDETTE-28 | ethinyl estradiol; levonorgestrel | TABLET;ORAL-28 | 018782-001 | Jul 21, 1982 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | |||||
| Teva Branded Pharm | VANTRELA ER | hydrocodone bitartrate | TABLET, EXTENDED RELEASE;ORAL | 207975-005 | Jan 17, 2017 | DISCN | Yes | No | 8,445,018 | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Teva Branded Pharm | LOESTRIN FE 1/20 | ethinyl estradiol; norethindrone acetate | TABLET;ORAL-28 | 017354-001 | Approved Prior to Jan 1, 1982 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Teva Branded Pharm
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Teva Branded Pharm | LOXITANE C | loxapine hydrochloride | CONCENTRATE;ORAL | 017658-001 | Approved Prior to Jan 1, 1982 | 3,546,226 | ⤷ Sign Up |
| Teva Branded Pharm | SEASONALE | ethinyl estradiol; levonorgestrel | TABLET;ORAL | 021544-001 | Sep 5, 2003 | 5,898,032 | ⤷ Sign Up |
| Teva Branded Pharm | QVAR 80 | beclomethasone dipropionate | AEROSOL, METERED;INHALATION | 020911-001 | Sep 15, 2000 | 5,683,677 | ⤷ Sign Up |
| Teva Branded Pharm | NORDETTE-28 | ethinyl estradiol; levonorgestrel | TABLET;ORAL-28 | 018782-001 | Jul 21, 1982 | 3,959,322 | ⤷ Sign Up |
| Teva Branded Pharm | PROAIR DIGIHALER | albuterol sulfate | POWDER, METERED;INHALATION | 205636-002 | Dec 21, 2018 | 6,701,917 | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for TEVA BRANDED PHARM drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 0.1 mg/0.02 mg and 0.01 mg | ➤ Subscribe | 2009-11-16 |
| ➤ Subscribe | Tablets | 0.15 mg/0.03 mg | ➤ Subscribe | 2004-03-29 |
| ➤ Subscribe | Tablets | 1 mg/0.02 mg and 75 mg | ➤ Subscribe | 2006-04-17 |
| ➤ Subscribe | Tablets | 0.15 mg/0.02 mg, 0.15 mg/0.025 mg, 0.15 mg/0.03 mg and 0.01 mg | ➤ Subscribe | 2013-07-10 |
| ➤ Subscribe | Tablets | 0.15 mg/0.03 mg/0.01 mg | ➤ Subscribe | 2008-01-22 |
International Patents for Teva Branded Pharm Drugs
Supplementary Protection Certificates for Teva Branded Pharm Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1602370 | 09C0020 | France | ⤷ Sign Up | PRODUCT NAME: COMBINAISON COMRENANT L’ALISKIREN SOUS FORME DE BAE LIBRE OU UN SEL DE CELUI-CI PHARMACEUTIQUEMENT ACCEPTABLE, ET L’HYDROCHLOROTHIAZIDE OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI; REGISTRATION NO/DATE IN FRANCE: EU/1/08/491/001 DU 20090116; REGISTRATION NO/DATE AT EEC: 58935 01-04 DU 20081028 |
| 1602370 | SPC/GB09/024 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: COMBINATION COMPRISING ALISKIREN, AS THE FREE BASE OR AS A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, AND HYDROCHLOROTHIAZIDE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REGISTERED: CH 5893501 20081028; CH 5893502 20081028; CH 5893503 20081028; CH 5893504 20081028; UK EU/1/08/491/006 20090116; UK EU/1/08/491/002 20090116; UK EU/1/08/491/003 20090116; UK EU/1/08/491/004 20090116; UK EU/1/08/491/005 20090116; UK EU/1/08/491/007 20090116; UK EU/1/08/491/080 20090116; UK EU/1/08/491/074 20090116; UK EU/1/08/491/075 20090116; UK EU/1/08/491/076 20090116; UK EU/1/08/491/077 20090116; UK EU/1/08/491/078 20090116; UK EU/1/08/491/079 20090116; UK EU/1/08/491/068 20090116; UK EU/1/08/4 |
| 1453521 | 15C0050 | France | ⤷ Sign Up | PRODUCT NAME: ETHINYLESTRADIOL ET MELANGE DE LEVONORGESTREL ET ETHINYLESTRADIOL; NAT. REGISTRATION NO/DATE: NL 42237 20150320; FIRST REGISTRATION: SK - 17/0017/15-S 20150129 |
| 1507558 | 12C0033 | France | ⤷ Sign Up | PRODUCT NAME: ALISKIRENE OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE E CELUI-CI, AMLODIPINE OU SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI, ET HYDROCHLOROTHIAZIDE OU SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI; NAT. REGISTRATION NO/DATE: EU/1/11/730/001 20111122; FIRST REGISTRATION: CH - 6167801 20110705 |
| 0771217 | 07C0001 | France | ⤷ Sign Up | PRODUCT NAME: ETHINYLESTRADIOL BETADEX CLATHRATE; NAT. REGISTRATION NO/DATE: NL 32343 20060710; FIRST REGISTRATION: NL - RVG 31781 20050804 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.