Sanofi Aventis Us Company Profile
✉ Email this page to a colleague
What is the competitive landscape for SANOFI AVENTIS US, and when can generic versions of SANOFI AVENTIS US drugs launch?
SANOFI AVENTIS US has one hundred and twenty-two approved drugs.
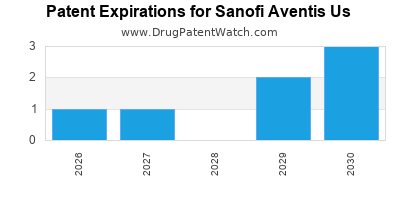
There are ten US patents protecting SANOFI AVENTIS US drugs.
There are two hundred and seventeen patent family members on SANOFI AVENTIS US drugs in fifty-two countries and one hundred and fifteen supplementary protection certificates in nineteen countries.
Summary for Sanofi Aventis Us
| International Patents: | 217 |
| US Patents: | 10 |
| Tradenames: | 97 |
| Ingredients: | 85 |
| NDAs: | 122 |
Drugs and US Patents for Sanofi Aventis Us
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sanofi Aventis Us | ELOXATIN | oxaliplatin | INJECTABLE;INTRAVENOUS | 021759-001 | Jan 31, 2005 | AP | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sanofi Aventis Us | LASIX | furosemide | INJECTABLE;INJECTION | 016363-001 | Approved Prior to Jan 1, 1982 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sanofi Aventis Us | PENETREX | enoxacin | TABLET;ORAL | 019616-004 | Dec 31, 1991 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sanofi Aventis Us | LOVENOX (PRESERVATIVE FREE) | enoxaparin sodium | INJECTABLE;SUBCUTANEOUS | 020164-002 | Jan 30, 1998 | AP | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sanofi Aventis Us | TACE | chlorotrianisene | CAPSULE;ORAL | 008102-004 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sanofi Aventis Us | PHISO-SCRUB | hexachlorophene | SPONGE;TOPICAL | 017446-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sanofi Aventis Us | DEMI-REGROTON | chlorthalidone; reserpine | TABLET;ORAL | 015103-002 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Sanofi Aventis Us
Paragraph IV (Patent) Challenges for SANOFI AVENTIS US drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Injection | 5 mg/mL, 40 mL vial | ➤ Subscribe | 2011-03-23 |
| ➤ Subscribe | Extended-release Tablets | 6.25 mg | ➤ Subscribe | 2006-02-24 |
| ➤ Subscribe | Tablets | 7 mg and 14 mg | ➤ Subscribe | 2016-09-12 |
| ➤ Subscribe | Tablets | 300 mg/25 mg | ➤ Subscribe | 2006-06-06 |
| ➤ Subscribe | Tablets | 400 mg | ➤ Subscribe | 2013-07-01 |
| ➤ Subscribe | Injection | 5 mg/mL, 10 mL and 20 mL vials | ➤ Subscribe | 2007-02-09 |
| ➤ Subscribe | Injection | 200 mg/40 mL | ➤ Subscribe | 2007-07-16 |
| ➤ Subscribe | Injection | 100 mg/mL, 3 mL vials | ➤ Subscribe | 2006-12-07 |
| ➤ Subscribe | Extended-release Tablets | 12.5 mg | ➤ Subscribe | 2006-01-19 |
| ➤ Subscribe | Tablets | 150 mg/12.5 mg and 300 mg/12.5 mg | ➤ Subscribe | 2004-11-10 |
| ➤ Subscribe | Tablets | 75 mg, 150 mg and 300 mg | ➤ Subscribe | 2004-05-25 |
| ➤ Subscribe | Tablets | 300 mg | ➤ Subscribe | 2009-03-04 |
| ➤ Subscribe | Injection | 40 mg/mL, 0.5 mL and 2 mL vials | ➤ Subscribe | 2009-06-30 |
| ➤ Subscribe | For Injection | 50 mg/vial and 100 mg/vial | ➤ Subscribe | 2007-02-09 |
International Patents for Sanofi Aventis Us Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| New Zealand | 598744 | ⤷ Try a Trial |
| Nicaragua | 201000172 | ⤷ Try a Trial |
| Portugal | 2477611 | ⤷ Try a Trial |
| Taiwan | I533866 | ⤷ Try a Trial |
| Tunisia | SN06086 | ⤷ Try a Trial |
| Japan | 2013509394 | ⤷ Try a Trial |
| South Korea | 101547880 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Sanofi Aventis Us Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2344130 | 300884 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: OZENOXACIN; REGISTRATION NO/DATE: ES/H/414/1/DC 20170519 |
| 0454511 | SPC/GB99/008 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: 2-N-BUTYL-4-SPIROCYCLOPENTANE-1-((2'-(TETRAZOL-5-YL)BIPHENYL-4-YL)METHYL)-2-IMIDAZOLIN-5-ONE)(GENERIC NAME IRBESARTAN) OPTIONALLY IN THE FORM OF ONE OF ITS SALTS AND HYDROCHLOROTHIAZIDE; REGISTERED: UK EU/1/98/086/001 19981015; UK EU/1/98/086/002 19981015; UK EU/1/98/086/003 19981015; UK EU/1/98/086/004 19981015; UK EU/1/98/086/005 19981015; UK EU/1/98/086/006 19981015 |
| 1744764 | 122018000134 | Germany | ⤷ Try a Trial | PRODUCT NAME: ZUSAMMENSETZUNG AUS DAUNORUBICIN UND CYTARABIN; REGISTRATION NO/DATE: EU/1/18/1308 20180823 |
| 1381356 | C300644 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: TERIFLUONOMIDE, ITS STEROISOMER AND PHARAMCEUTICALLY ACCEPTABLE SALTS THEREOF; REGISTRATION NO/DATE: EU/1/13/838/001-005 20130826 |
| 2344130 | 132017000093887 | Italy | ⤷ Try a Trial | PRODUCT NAME: OZENOXACIN(DUBINE); AUTHORISATION NUMBER(S) AND DATE(S): ES/H/414/01/DC, 20170519;045237017, 20170711 |
| 3300601 | 2290030-2 | Sweden | ⤷ Try a Trial | PRODUCT NAME: COMBINATION OF DAUNORUBICIN AND CYTARABINE; REG. NO/DATE: EU/1/18/1308 20180827 |
| 1381356 | C 2014 006 | Romania | ⤷ Try a Trial | PRODUCT NAME: TERIFLUNOMIDA, STEREOIZOMERUL SAU SI SARURILE FARMACEUTICACCEPTABILE ALEACESTEIA; NATIONAL AUTHORISATION NUMBER: EU/1/13/838/001, EU/1/13/838/002, EU/1/13/838/003, EU/1/13/838/004, EU/1/13/838/005; DATE OF NATIONAL AUTHORISATION: 20130826; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/13/838/001, EU/1/13/838/002, EU/1/13/838/003, EU/1/13/838/004, EU/1/13/838/005; DATE OF FIRST AUTHORISATION IN EEA: 20130826 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.