Astrazeneca Ab Company Profile
✉ Email this page to a colleague
What is the competitive landscape for ASTRAZENECA AB, and what generic alternatives to ASTRAZENECA AB drugs are available?
ASTRAZENECA AB has twelve approved drugs.
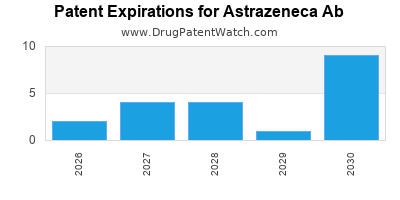
There are fifty-six US patents protecting ASTRAZENECA AB drugs. There is one tentative approval on ASTRAZENECA AB drugs.
There are nine hundred and six patent family members on ASTRAZENECA AB drugs in fifty-four countries and one hundred and forty-two supplementary protection certificates in nineteen countries.
Summary for Astrazeneca Ab
| International Patents: | 906 |
| US Patents: | 56 |
| Tradenames: | 13 |
| Ingredients: | 10 |
| NDAs: | 12 |
| Drug Master File Entries: | 2 |
| Patent Litigation for Astrazeneca Ab: | See patent lawsuits for Astrazeneca Ab |
| PTAB Cases with Astrazeneca Ab as patent owner: | See PTAB cases with Astrazeneca Ab as patent owner |
Drugs and US Patents for Astrazeneca Ab
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astrazeneca Ab | BYDUREON BCISE | exenatide synthetic | SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 209210-001 | Oct 20, 2017 | RX | Yes | Yes | 8,329,648*PED | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Astrazeneca Ab | QTERN | dapagliflozin; saxagliptin hydrochloride | TABLET;ORAL | 209091-002 | May 2, 2019 | RX | Yes | No | 8,501,698 | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Astrazeneca Ab | QTERNMET XR | dapagliflozin; metformin hydrochloride; saxagliptin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 210874-004 | May 2, 2019 | DISCN | Yes | No | 8,716,251 | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Astrazeneca Ab | FARXIGA | dapagliflozin | TABLET;ORAL | 202293-002 | Jan 8, 2014 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Astrazeneca Ab
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Astrazeneca Ab | BYDUREON BCISE | exenatide synthetic | SUSPENSION, EXTENDED RELEASE;SUBCUTANEOUS | 209210-001 | Oct 20, 2017 | 6,936,590 | ⤷ Sign Up |
| Astrazeneca Ab | FARXIGA | dapagliflozin | TABLET;ORAL | 202293-001 | Jan 8, 2014 | 7,741,269 | ⤷ Sign Up |
| Astrazeneca Ab | BYETTA | exenatide synthetic | INJECTABLE;SUBCUTANEOUS | 021773-002 | Apr 28, 2005 | 6,902,744 | ⤷ Sign Up |
| Astrazeneca Ab | SYMLIN | pramlintide acetate | INJECTABLE;SUBCUTANEOUS | 021332-001 | Mar 16, 2005 | 7,271,238 | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for ASTRAZENECA AB drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | HydrochlorideExtended-release Tablets | 2.5 mg/1000 mg | ➤ Subscribe | 2018-10-29 |
| ➤ Subscribe | Extended-release Tablets | 5 mg/500 mg, 2.5 mg/1000 mg, and 5 mg/1000 mg | ➤ Subscribe | 2013-07-31 |
| ➤ Subscribe | Tablets | 2.5 mg and 5 mg | ➤ Subscribe | 2013-07-31 |
| ➤ Subscribe | Injection | 250 mg/mL, 1.2 mL and 2.4 mL prefilled syringe | ➤ Subscribe | 2014-06-11 |
International Patents for Astrazeneca Ab Drugs
Supplementary Protection Certificates for Astrazeneca Ab Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1506211 | CR 2014 00037 | Denmark | ⤷ Sign Up | PRODUCT NAME: ET KOMBINATIONSPRODUKT AF DAPAGLIFLOZIN ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF, HERUNDER DAPAGLIFLOZINPROPANDIOLMONOHYDRAT OG METFORMIN ELLER SALTE DERAF, HERUNDER METFORMINHYDROCHLORID; REG. NO/DATE: EU/1/13/900 20140116 |
| 2435024 | 2021019 | Norway | ⤷ Sign Up | PRODUCT NAME: KOMBINASJON AV FORMOTEROL; REG. NO/DATE: EU/1/20/1498 20201216 |
| 2498758 | PA2020003 | Lithuania | ⤷ Sign Up | PRODUCT NAME: METFORMINAS ARBA FARMACINIU POZIURIU PRIIMTINA JO DRUSKA; SAKSAGLIPTINAS ARBA FARMACINIU POZIURIU PRIIMTINA JO DRUSKA; DAPAGLIFLOZINAS ARBA FARMACINIU POZIURIU PRIIMTINAS JO SOLVATAS; REGISTRATION NO/DATE: EU/1/19/1401 20191111 |
| 1532149 | C300569 | Netherlands | ⤷ Sign Up | PRODUCT NAME: LINAGLIPTINE, DESGEWENST IN DE VORM VAN EEN ZOUT, IN COMBINATIE MET METFORMINEHYDROCHLORIDE; REGISTRATION NO/DATE: EU/1/12/780/001-028 20120720 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.