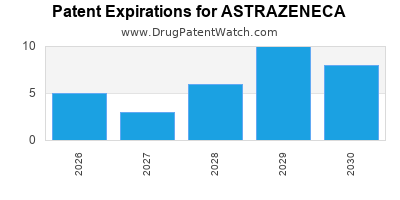
ASTRAZENECA Company Profile
✉ Email this page to a colleague
What is the competitive landscape for ASTRAZENECA, and what generic alternatives to ASTRAZENECA drugs are available?
ASTRAZENECA has ninety-three approved drugs.
There are one hundred and twenty-four US patents protecting ASTRAZENECA drugs. There is one tentative approval on ASTRAZENECA drugs.
There are two thousand one hundred and ninety-two patent family members on ASTRAZENECA drugs in seventy countries and three hundred and forty-six supplementary protection certificates in twenty countries.
Summary for ASTRAZENECA
| International Patents: | 2192 |
| US Patents: | 124 |
| Tradenames: | 66 |
| Ingredients: | 60 |
| NDAs: | 93 |
| Patent Litigation for ASTRAZENECA: | See patent lawsuits for ASTRAZENECA |
| PTAB Cases with ASTRAZENECA as patent owner: | See PTAB cases with ASTRAZENECA as patent owner |
Drugs and US Patents for ASTRAZENECA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astrazeneca | TAGRISSO | osimertinib mesylate | TABLET;ORAL | 208065-001 | Nov 13, 2015 | RX | Yes | No | 9,732,058 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Astrazeneca | EPANOVA | omega-3-carboxylic acids | CAPSULE;ORAL | 205060-001 | May 5, 2014 | DISCN | Yes | No | 9,050,309 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Astrazeneca Ab | XIGDUO XR | dapagliflozin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 205649-001 | Oct 29, 2014 | RX | Yes | No | 8,501,698 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ASTRAZENECA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Astrazeneca | NEXIUM | esomeprazole magnesium | CAPSULE, DELAYED REL PELLETS;ORAL | 021153-002 | Feb 20, 2001 | 4,508,905 | ⤷ Try a Trial |
| Astrazeneca Ab | QTERN | dapagliflozin; saxagliptin hydrochloride | TABLET;ORAL | 209091-001 | Feb 27, 2017 | RE44186 | ⤷ Try a Trial |
| Astrazeneca Ab | SYMLIN | pramlintide acetate | INJECTABLE;SUBCUTANEOUS | 021332-001 | Mar 16, 2005 | 7,407,934 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for ASTRAZENECA drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Inhalation Suspension | 0.25 mg/2 mL and 0.5 mg/2 mL | ➤ Subscribe | 2005-09-15 |
| ➤ Subscribe | Tablets | 2.5 mg and 5 mg | ➤ Subscribe | 2013-07-31 |
| ➤ Subscribe | Delayed-release Capsules | 20 mg | ➤ Subscribe | 2007-03-19 |
| ➤ Subscribe | Extended-release Tablets | 400 mg | ➤ Subscribe | 2008-06-18 |
| ➤ Subscribe | Tablets | 200 mg and 300 mg | ➤ Subscribe | 2008-06-12 |
| ➤ Subscribe | Tablets | 90 mg | ➤ Subscribe | 2015-07-20 |
| ➤ Subscribe | Tablets | 500 mcg | ➤ Subscribe | 2015-03-02 |
| ➤ Subscribe | Nasal Spray | 0.032 mg (32 mcg)/spray | ➤ Subscribe | 2007-05-14 |
| ➤ Subscribe | Tablets | 50 mg, 150 mg and 400 mg | ➤ Subscribe | 2007-02-12 |
| ➤ Subscribe | Delayed-release for Oral Suspension | 2.5 mg and 5 mg | ➤ Subscribe | 2018-09-24 |
| ➤ Subscribe | Injection | 250 mg/mL, 1.2 mL and 2.4 mL prefilled syringe | ➤ Subscribe | 2014-06-11 |
| ➤ Subscribe | Injection | 50 mg/mL, 2.5 mL and 5 mL syringe | ➤ Subscribe | 2009-10-01 |
| ➤ Subscribe | HydrochlorideExtended-release Tablets | 2.5 mg/1000 mg | ➤ Subscribe | 2018-10-29 |
| ➤ Subscribe | Inhalation Suspension | 1 mg/2 mL | ➤ Subscribe | 2010-05-28 |
| ➤ Subscribe | Delayed-release Tablets | 20 mg | ➤ Subscribe | 2012-03-30 |
| ➤ Subscribe | Extended-release Tablets | 150 mg | ➤ Subscribe | 2008-11-17 |
| ➤ Subscribe | Extended-release Tablets | 50 mg | ➤ Subscribe | 2008-10-17 |
| ➤ Subscribe | Tablets | 60 mg | ➤ Subscribe | 2015-09-30 |
| ➤ Subscribe | Tablets | 25 mg | ➤ Subscribe | 2005-08-12 |
| ➤ Subscribe | Delayed-release | 20 mg and 40 mg | ➤ Subscribe | 2005-08-05 |
| ➤ Subscribe | Tablets | 100 mg, 200 mg and 300 mg | ➤ Subscribe | 2006-02-21 |
| ➤ Subscribe | For Injection | 20 mg/vial and 40 mg/vial | ➤ Subscribe | 2009-11-23 |
| ➤ Subscribe | Extended-release Tablets | 5 mg/500 mg, 2.5 mg/1000 mg, and 5 mg/1000 mg | ➤ Subscribe | 2013-07-31 |
International Patents for ASTRAZENECA Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Brazil | 122017015091 | ⤷ Try a Trial |
| China | 101360718 | ⤷ Try a Trial |
| Canada | 2929956 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for ASTRAZENECA Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2435025 | 2019026 | Norway | ⤷ Try a Trial | PRODUCT NAME: KOMBINASJON AV GLYKOPYRROLAT (INKLUDERT EVENTUELLE FARMASOEYTISK AKSEPTABLE SALTER, ESTERE ELLER ENANTIOMERER DERAV) OG FORMOTEROL (INNBEFATTENDE HVILKE SOM HELST FARMASOEYTISK AKSEPTABLE SALTER, ESTERE ELLER ENANTIOMERER DERAV); REG. NO/DATE: EU/1/18/1339 20190104 |
| 0823900 | C300429 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: GEFITINIB, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT; REGISTRATION NO/DATE: EU/1/09/526/00156154 2009020624 |
| 1482932 | 1990010-9 | Sweden | ⤷ Try a Trial | PRODUCT NAME: BINIMETINIB AND PHARMACEUTICALLY ACCEPTABLE SALTS AND SOLVATES THEROF; REG. NO/DATE: EU/1/18/1315 20180924 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.