In the world of healthcare, pharmaceutical procurement stands as a cornerstone, profoundly influencing everything from drug accessibility and patient safety to the financial viability of healthcare systems. It’s far more than just “buying drugs”; it’s a strategic dance between need, quality, cost, and compliance, ultimately aimed at transforming data into market domination. Think of it like a highly skilled orchestra conductor, where every section (planning, sourcing, contracting, and managing) must play in perfect harmony to produce a masterful performance.
Understanding the Core of Pharmaceutical Procurement
At its heart, pharmaceutical procurement is the systematic process of acquiring medicines, vaccines, and related medical supplies. Its fundamental objective is to ensure the timely supply of the right pharmaceuticals, in the right quantity, meeting approved quality standards, all while securing the most favorable purchase price. This complex undertaking demands a multifaceted skill set, encompassing pharmaceutical knowledge, managerial prowess, legal acumen, and financial expertise. Without these diverse capabilities, the entire process can falter, leading to stockouts, compromised patient care, or inflated costs that cripple healthcare budgets.
The Evolving Landscape of Pharma Procurement
The pharmaceutical procurement landscape is in a constant state of flux, shaped by technological advancements, evolving ethical considerations, a significant shift towards value-based procurement, and the ever-present demands of global supply chain management. We’re seeing a rapid adoption of technologies like artificial intelligence (AI), blockchain, and robotics, which are reshaping how procurement operations are managed. This dynamism necessitates an agile and adaptable approach, moving beyond traditional transactional models to embrace strategic partnerships and data-driven decision-making.
Key Considerations in Pharmaceutical Procurement
Navigating the complexities of pharmaceutical procurement requires a keen awareness of several critical factors. These aren’t merely checkboxes; they are foundational pillars upon which successful procurement strategies are built.
Drug Selection and Quantity Determination
The first step in effective procurement is accurately identifying what drugs are needed and how much of each. This involves a rigorous review of drug selections, aligning needs with budgetary constraints, and leveraging consumption data to forecast future demand. Imagine trying to build a house without a blueprint or a precise list of materials; it’s a recipe for disaster. Similarly, in pharma, inaccurate forecasting can lead to either costly overstocking or dangerous shortages.
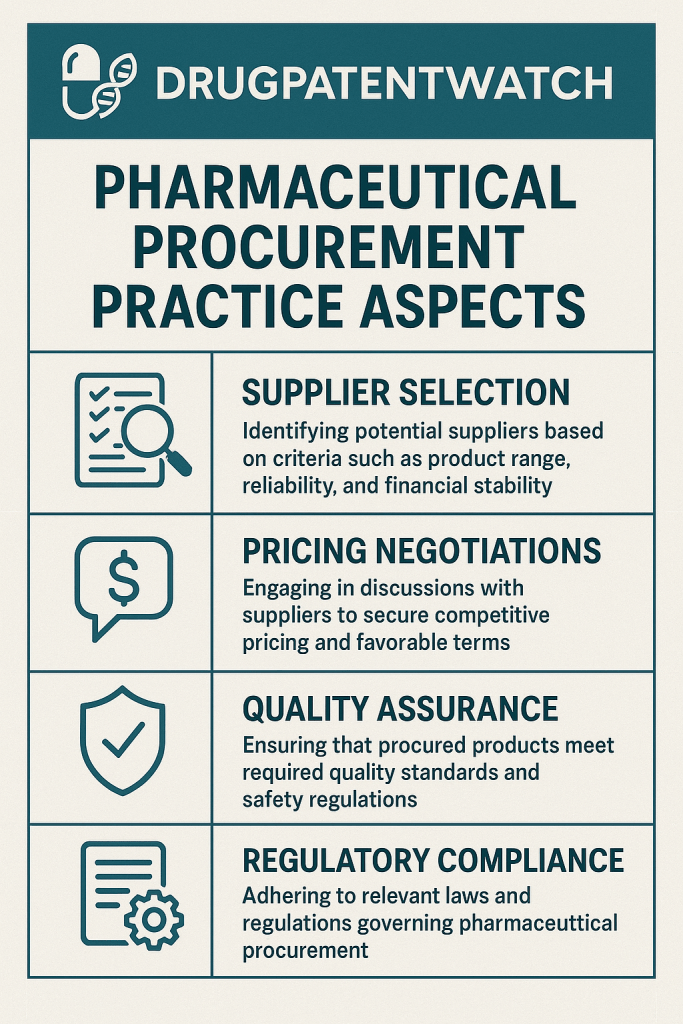
Procurement Methods and Supplier Selection
Once needs are identified, the next critical phase involves selecting the most appropriate procurement method and choosing reliable suppliers. Common procurement methods include restricted tenders, open tenders, direct procurement, competitive negotiation, and international or local shopping. Each method has its advantages and disadvantages, and the choice often depends on the specific drug, market conditions, and urgency. Supplier prequalification is paramount, involving thorough checks on past performance, manufacturing practices, and financial viability. As industry expert Dr. Elena Petrova, Head of Global Pharmaceutical Supply Chains at a leading NGO, often states, “A pharmaceutical supply chain is only as strong as its weakest link, and often, that link is an unvetted supplier.”
Contract Terms and Order Tracking
Beyond selection, the meticulous outlining of contract terms and robust order status tracking are vital. Clear contracts protect both buyer and seller, detailing quality standards, delivery timelines, payment schedules, and dispute resolution mechanisms. Real-time tracking ensures transparency and allows for proactive intervention in case of delays or issues.
Receiving, Checking, and Distribution
The procurement process doesn’t end with a signed contract. The careful receiving and checking of drugs upon arrival are critical to ensure they meet specified quality standards and quantities. Any discrepancies must be addressed immediately. Finally, efficient distribution within the healthcare system ensures that medicines reach patients when and where they are needed, completing the procurement cycle.
Regulatory Frameworks: The Unseen Hand of Procurement
Pharmaceutical procurement operates within a tightly regulated environment, often described as a labyrinth of rules and guidelines. These comprehensive regulatory requirements significantly influence procurement practices, necessitating thorough supplier qualification processes, detailed documentation requirements, and ongoing compliance monitoring throughout the supplier relationship lif1ecycle. Regulatory compliance is not just a checkbox exercise; it is a critical business imperative that directly impacts patient safety, product quality, and a company’s market standing.
Navigating Regulatory Complexities
The pharmaceutical industry faces stringent regulatory requirements from various bodies such as the FDA (US), EMA (Europe), CDSCO (India), and WHO-GMP (Good Manufacturing Practices) guidelines. Staying updated with evolving regulations across these diverse frameworks is a continuous challenge. For instance, regulatory inspections increased by 17.6% between 2022 and 2023, underscoring the heightened scrutiny on the industry. Furthermore, the pharmaceutical industry has historically paid billions in financial penalties for poor manufacturing practices, highlighting the dire consequences of non-compliance.
Key Regulatory Impacts
Effective compliance in pharma procurement delivers three essential outcomes:
- Risk Mitigation: Prevention of regulatory penalties and product recalls, safeguarding both financial stability and patient trust.
- Supply Chain Integrity: Assurance of product quality and patient safety, ensuring that what reaches the patient is safe and effective.
- Market Acceleration: Faster time-to-market with full regulatory confidence, providing a competitive edge in a dynamic industry.
Non-compliant vendors can trigger audit failures, leading to batch rejections and production delays, creating a ripple effect throughout the supply chain. Global buyers must navigate multiple regulatory frameworks, adding layers of complexity to supplier selection and management.
Good Manufacturing Practices (GMP)
GMP guidelines are quality-focused standards that profoundly impact supplier selection. Suppliers must demonstrate adherence to these practices to ensure the quality and safety of raw materials and finished products. This often involves extensive audits and ongoing monitoring.
International Standards and Quality Systems
Standards like ICH Q10 (Pharmaceutical Quality System) and ISO 9001 (Quality Management Systems) influence how vendors operate and are audited. These frameworks provide a systematic approach to managing quality, from raw material sourcing to product distribution.
Documentation and Approval Trails
Missing documentation for purchase orders or a lack of approval trails for deviations can lead to regulatory penalties and costly remediation. Digital solutions that centralize documentation and automate approval workflows are becoming indispensable in addressing these pitfalls.
Optimizing Pharmaceutical Supply Chains: A Path to Efficiency
An optimized pharmaceutical supply chain is not merely about moving products from point A to point B; it’s about building resilience, enhancing visibility, and leveraging technological advancements to achieve unparalleled efficiency and security. It’s akin to a finely tuned logistics network, where every component works seamlessly to deliver critical medicines.
Supply Chain Visibility and Transparency2
Visibility refers to the ability to track and trace products throughout the supply chain, while transparency involves sharing information with stakeholders to build trust and ensure compliance. The 3pharmaceutical supply chain is inherently vulnerable to risks such as counterfeiting, diversion, and product tampering. Enhancing visibility and transparency can significantly mitigate these risks by enabling real-time monitoring and tracking of products.
Tools like RFID, GPS tracking, and barcode scanning allow for accurate and efficient product tracking. More advanced technologies like blockchain provide a secure and transparent way to record transactions and track products from their origin to the patient.
Advanced Technologies for Optimization
The adoption of emerging technologies is transforming pharmaceutical supply chains:
- Artificial Intelligence (AI) and Machine Learning (ML): AI-powered predictive analytics helps forecast demand more accurately and optimize inventory management, reducing waste and ensuring availability. AI can also enhance real-time monitoring and secure pharma supply chains by detecting irregularities and possible risks.
- Internet of Things (IoT): IoT devices can be used to track and monitor products in real-time, providing greater visibility and control over conditions like temperature and humidity, which are crucial for many pharmaceutical products.
- Robotics and Automation: These technologies improve efficiency and accuracy in logistics operations, such as warehousing and order fulfillment, reducing manual errors and speeding up processes.
- E-Procurement Platforms: These digital platforms reduce manual workflows, automate purchase order generation and approval, and enforce regulatory compliance, leading to reduced procurement cycle times and increased transparency.
Collaboration and Risk Management
Optimizing pharmaceutical supply chains also requires robust collaboration and proactive risk management. This involves working closely with stakeholders—manufacturers, distributors, regulatory agencies, and suppliers—to share information, coordinate operations, and collectively mitigate risks.
- Supplier Diversification: Reducing dependence on a single supplier mitigates the risk of supply chain disruptions caused by unforeseen events like natural disasters or geopolitical instability.
- Contingency Planning: Developing comprehensive contingency plans helps organizations respond effectively to disruptions, minimizing their impact on product availability.
- Regular Risk Assessments: Identifying potential risks and vulnerabilities through regular assessments is crucial for developing targeted mitigation strategies.
Strategic Sourcing and Supplier Relationship Management
The bedrock of successful pharmaceutical procurement lies in strategic sourcing and the cultivation of strong, collaborative supplier relationships. It’s about moving beyond transactional interactions to forge long-term partnerships that drive mutual value and resilience.
The Importance of Strategic Sourcing
Strategic sourcing is a systematic approach to acquiring goods and services that aligns with an organization’s long-term goals and objectives. It involves analyzing market conditions, identifying cost-saving opportunities, and establishing supplier relationships to secure favorable term4s. It goes beyond simple cost-cutting to encompass supply chain efficiency, innovation, and sustainability.
For instance, by shifting to global sourcing strategies, companies can diversify their supplier base and explore more cost-effective options. Negotiating volume discounts with preferred suppliers and even introducing reverse auctions for non-critical items are examples of strategic approaches to cost reduction.
Building Strong Supplier Relationships
Developing long-term partnerships with reliable suppliers is a cornerstone of effective pharmaceutical procurement. These relationships foster transparency in communication, leading to quicker resource allocation, new perspectives, and insights into changing market trends.
- Supplier Performance Management (SPM) Systems: Integrating SPM systems allows for real-time monitoring of supplier performance against agreed-upon metrics, identifying potential issues before they escalate.
- Pre-qualification and Audits: A robust pre-qualification process ensures suppliers meet stringent quality, ethical, and financial criteria. Regular audits verify ongoing compliance and performance.
- Collaborative Innovation: Strong partnerships can lead to collaborative innovation, where suppliers and buyers work together to develop new solutions, improve processes, or address shared challenges.
Cost Management and Value-Based Procurement
In an industry where drug prices and healthcare costs are under constant scrutiny, effective cost management in pharmaceutical procurement is paramount. However, the focus is shifting from simply cutting costs to embracing value-based procurement, prioritizing outcomes and overall value over the lowest upfront price.
Strategies for Cost Reduction
While value is key, cost reduction remains a vital aspect. Organizations employ various strategies:
- Volume Discounts: Leveraging purchasing power to negotiate favorable terms and discounts for larger quantities of drugs.
- Global Sourcing: Exploring international markets to find competitive pricing and diversify the supplier base.
- Group Purchasing Organizations (GPOs): Collaborating with other healthcare entities through GPOs to aggregate demand and secure better pricing.
- Generic Drug Utilization: Actively promoting the use of generic drugs once brand-name drug patents expire. Generics are chemically identical to their brand-name counterparts but are often significantly cheaper, driving competition and lowering costs. This is where tools like DrugPatentWatch become invaluable, providing insights into patent expiration dates and upcoming generic opportunities.
The Shift to Value-Based Procurement
Value-based procurement focuses on the total value delivered by a pharmaceutical product or service, considering not just the acquisition cost but also its impact on patient outcomes, operational efficiency, and long-term savings. It’s a holistic view that challenges the traditional cost-centric model.
- Outcome-Oriented Metrics: Shifting focus from price per unit to metrics like reduced hospital readmissions, improved patient adherence, or shorter treatment durations.
- Lifecycle Costing: Evaluating the total cost of a drug over its entire lifecycle, including administration, monitoring, and potential side effects.
- Supplier Innovation Incentives: Encouraging suppliers to propose innovative solutions that deliver better value, even if the initial cost is higher.
Risk Management in Pharmaceutical Procurement
The pharmaceutical supply chain is inherently exposed to a multitude of risks, from supply disruptions and quality failures to geopolitical instability and cyber threats. Proactive and comprehensive risk management is not just good practice; it’s a non-negotiable imperative.
Identifying and Assessing Risks
Risk management begins with systematically identifying what can go wrong. This includes:
- Supply Chain Disruptions: Natural disasters, pandemics, political unrest, or manufacturing issues at a supplier’s facility can halt the flow of essential medicines. For example, a global pandemic could significantly impact raw material suppliers located overseas.
- Quality and Safety Risks: Substandard ingredients, manufacturing errors, or improper storage can compromise drug quality and patient safety.
- Regulatory and Compliance Risks: Non-adherence to evolving regulations can lead to fines, product recalls, and reputational damage.
- Financial Risks: Price volatility, currency fluctuations, or supplier insolvency can impact procurement budgets.
- Geopolitical Risks: Trade wars, tariffs, or political instability in key sourcing regions can disrupt supply and increase costs.
Mitigation and Contingency Planning
Once risks are identified, strategies for mitigation and contingency planning are developed. This involves:
- Supplier Due Diligence: Thorough assessment of potential suppliers’ financial stability, quality systems, and risk management capabilities before entering into a relationship.
- Diversification of Sourcing: Spreading procurement across multiple suppliers and geographical regions to reduce dependence on any single source.
- Inventory Management Strategies: Implementing robust inventory control systems to balance supply and demand, including maintaining safety stock for critical medicines.
- Supply Chain Mapping and Transparency: Gaining end-to-end visibility into the supply chain to identify potential bottlenecks and vulnerabilities.
- Crisis Response Plans: Developing detailed plans for responding to various types of disruptions, including communication protocols, alternative sourcing options, and expedited logistics.
“Quality risk management is a systematic process for assessing, controlling, communicating, and reviewing risks to the quality of the drug product across the product lifecycle.” — GMP SOP on Quality Risk Management
Ethical Considerations and Sustainability in Procurement
Beyond economic and operational considerations, ethical practices and sustainability are increasingly becoming central to pharmaceutical procurement. This reflects a broader societal expectation for corporations to operate responsibly and contribute positively to global well-being.
Ethical Sourcing and Practices
Ethical considerations span the entire procurement lifecycle, from raw material sourcing to fair labor practices. This includes:
- Fair Labor Practices: Ensuring suppliers adhere to fair labor standards, avoiding exploitation, child labor, and unsafe working conditions.
- Anti-Counterfeiting Measures: Prioritizing suppliers who employ robust anti-counterfeiting technologies and collaborate with regulatory authorities to combat illicit trade.
- Transparency and Accountability: Maintaining openness about sourcing, manufacturing processes, and pricing structures to build trust with patients and healthcare providers.
- Equitable Access: Considering the impact of procurement decisions on global health equity, striving for fair pricing and distribution to those in need, regardless of location or socioeconomic status.
Sustainable Procurement Initiatives
Sustainable procurement in pharmaceuticals aims to achieve value for money spent while generating benefits to society, the economy, and reducing environmental impact.
- Environmental Impact: Prioritizing suppliers with strong environmental management systems, focusing on reducing carbon footprint, waste, and water usage throughout the supply chain.
- Social Responsibility: Considering the social impact of procurement, including community engagement, ethical labor, and promoting diversity among suppliers. Takeda, for instance, has a comprehensive Sustainable Procurement program that aligns suppliers with their ESG (Environmental, Social, and Governance) practices, ensuring they operate with integrity, fairness, honesty, and perseverance. They are also members of the Pharmaceutical Supply Chain Initiative (PSCI), which leverages collective industry strength to make sustainable improvements.
- Circular Economy Principles: Exploring opportunities for reducing, reusing, and recycling packaging and materials within the pharmaceutical supply chain.
- Green Procurement: Preferring products and services that have a reduced environmental impact over their life cycle.
The Role of Technology in Modern Pharmaceutical Procurement
Technology is no longer a supporting player but a central character in the narrative of modern pharmaceutical procurement. Its transformative power is evident in every facet, from automating routine tasks to providing actionable insights for strategic decision-making.
Digital Transformation and E-Procurement
The digitalization of pharmaceutical procurement integrates digital technologies into the management of purchases. This aims to automate and optimize procurement, inventory management, and supplier relationship processes.
- E-Procurement Platforms: These platforms streamline the entire procure-to-pay cycle, from requisitioning and order placement to invoicing and payment. They reduce manual paperwork, cut errors, and provide a strategic overview of spending.
- Automated Workflows: Automation of tasks like purchase order generation, approvals, and compliance checks significantly increases efficiency and reduces cycle times.
- Digital Catalogs: Centralized digital catalogs allow buyers to easily browse and order from approved suppliers, ensuring compliance and negotiated pricing.
Leveraging AI, Machine Learning, and Blockchain
These advanced technologies are driving significant advancements:
- AI for Predictive Analytics: AI and machine learning algorithms enable accurate demand forecasting, supplier evaluation on multiple performance dimensions, and optimization of distribution networks. This allows for proactive decision-making rather than reactive problem-solving.
- Blockchain for Transparency and Traceability: Blockchain’s immutable, shared ledger establishes end-to-end supply chain transparency and provenance tracking. Every transaction, from raw material sourcing to product distribution, is recorded securely, helping to prevent counterfeiting and ensure drug integrity. Smart contracts on blockchain can automate actions when specific conditions are met, streamlining processes like quality checks and payments.
- Big Data Analytics: Analyzing vast datasets related to market trends, supplier performance, and consumption patterns provides actionable insights for strategic sourcing and risk management.
Global Trends Shaping Pharmaceutical Procurement
The pharmaceutical procurement landscape is constantly influenced by broader global trends, necessitating a forward-looking approach to remain competitive and resilient.
Rising Demand for Specialized Therapies
The surge in demand for specialized therapies, such as GLP-1s for obesity or advanced cell and gene therapies, is reshaping procurement strategies. These therapies often involve complex manufacturing processes, sensitive handling requirements, and specialized supply chains, requiring procurement teams to adapt quickly. The biopharmaceutical industry is projected to invest over $40 billion by 2030 to expand manufacturing capacity for these new modalities.
Geopolitical Shifts and Supply Chain Resilience
Geopolitical risks, such as trade tensions, conflicts, and protectionist policies, can significantly impact global supply chains. For example, legislative acts like the BIOSECURE Act aim to restrict certain foreign biotechnology firms, forcing pharmaceutical companies to re-evaluate their sourcing strategies and build more resilient, diversified supply chains to mitigate risks.
Inflationary Pressures and Cost Containment
Persistent inflationary pressures, stemming from rising costs of raw materials, transportation, and labor, are impacting profit margins. Pharmaceutical companies are responding by optimizing supply chains, negotiating favorable contracts, and exploring alternative sources to curb cost increases.
The AI Revolution in Procurement
Generative AI (GenAI) is poised to revolutionize procurement practices, with investments projected to grow at a CAGR of 32% from 2022 to 2032. GenAI can enhance various activities, including creating RFIs and RFPs, drafting supplier communications, identifying suppliers, and redlining contracts. This automation and intelligence can lead to a productivity boost of over 20% in procurement processes within the next 12-18 months.
Challenges and Solutions in Pharmaceutical Procurement
Despite advancements, pharmaceutical procurement faces persistent challenges that demand innovative solutions.
Common Challenges
- Lack of Supply Chain Transparency: This is a recurring issue, making it difficult to plan production, forecast demand, and manage inventory effectively. Without consistent data, regulators struggle to identify drug supply concerns, and healthcare providers can be blindsided by shortages.
- Poor Supplier Performance: Untimely deliveries, improper packaging, or a lack of appropriate documentation from suppliers can lead to unanticipated expenses and disruptions, impacting drug safety and availability.
- Unexpected Geographic Factors: Reliance on global markets makes companies vulnerable to geopolitical shifts, tariffs, and climate change, which can disrupt supplies and inflate costs.
- Drug Patent Limitations: While patents incentivize innovation by granting exclusive rights, their expiration opens the door to generic competition, which impacts pricing and market dynamics. Organizations like DrugPatentWatch provide valuable intelligence on drug patent status, helping procurement teams anticipate market shifts and generic opportunities.
Innovative Solutions
- Digital Transformation: Leveraging data and technology is key. This includes advanced analytics for better decision-making, e-procurement platforms for reducing manual workflows, and blockchain for establishing supply chain transparency and preventing counterfeiting.
- Strategic Partnerships: Developing long-term, collaborative relationships with suppliers can lead to more predictable supply, shared innovation, and improved risk mitigation.
- Proactive Risk Management Frameworks: Implementing systematic processes for identifying, assessing, and mitigating risks across the entire supply chain.
- Data-Driven Decision Making: Moving away from anecdotal evidence to decisions backed by real-time data, predictive models, and sophisticated analytics.
Drug Patents and Their Impact on Procurement
Drug patents play a pivotal role in the pharmaceutical ecosystem, directly influencing procurement strategies, drug pricing, and market access. Understanding their dynamics is crucial for any procurement professional.
The Purpose of Drug Patents
Patents are legal mechanisms that grant pharmaceutical companies exclusive rights to sell a drug they have developed for a certain period, typically 20 years from the filing date. This exclusivity is designed to incentivize the enormous investment required for drug research, development, and clinical trials. Without patent protection, companies would have little incentive to undertake the significant financial risks associated with bringing new medicines to market.
As a representative from the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) noted, “Patents provide the foundation for high-risk investment in new medicines.”
Impact on Procurement and Pricing
During the patent-protected period, the patent holder has a monopoly, allowing them to set drug prices based on recouping investment costs and market demand. This often results in higher prices for innovative, on-patent drugs. Procurement teams, therefore, face the challenge of balancing access to cutting-edge therapies with cost containment during this period.
Patent Expiration and Generic Opportunities
The true impact on procurement often comes into sharper focus as drug patents approach expiration. Once a patent expires, other manufacturers can produce “generic” versions of the drug. Generics are chemically identical to their brand-name counterparts and are rigorously tested to ensure bioequivalence. Because generic manufacturers do not incur the same research and development costs, they can offer these drugs at significantly lower prices, leading to increased competition and substantial cost savings for healthcare systems.
This is where intelligence tools like DrugPatentWatch become invaluable. DrugPatentWatch provides pharmaceutical global business intelligence, allowing procurement teams to monitor patent statuses, predict generic entries, and strategically plan for off-patent drug sourcing. WIPO’s Pat-INFORMED initiative also serves as a crucial resource, facilitating access to medicine patent information for procurement agencies and helping them make informed decisions. According to Mr. Wesley Kreft, Director, Global Supply Chain, i+solutions, “Pat-INFORMED will reduce the time required to go through the procurement process for medicines and thus improve the availability of medicines and help save lives.”
For example, when a blockbuster drug’s patent expires, procurement teams can shift from purchasing the expensive brand-name version to more affordable generics, generating significant savings that can be reinvested in other areas of healthcare. This shift also increases patient access to essential medicines.
Evergreening and Patent Challenges
Pharmaceutical companies sometimes employ strategies like “evergreening,” where they seek new patents on minor modifications to an existing drug, extending their market exclusivity. Procurement professionals need to be aware of these tactics and engage in robust legal and market analysis to understand the true patent landscape and identify opportunities for generic alternatives.
The Future of Pharmaceutical Procurement
The trajectory of pharmaceutical procurement points towards an increasingly digitized, data-driven, and patient-centric future, where agility and resilience will be paramount.
Enhanced Digitalization and Automation
We can expect a continued acceleration of digital transformation, with more sophisticated e-procurement platforms, widespread adoption of AI for complex decision-making, and robotic process automation (RPA) streamlining routine tasks. This will free up procurement professionals to focus on more strategic initiatives.
Predictive Analytics and AI-Driven Insights
The future will see procurement teams increasingly relying on predictive analytics, powered by AI and machine learning, to forecast demand, identify potential risks, and optimize inventory with unprecedented accuracy. This will enable truly proactive supply chain management.
Greater Emphasis on Traceability and Supply Chain Security
With the rise of e-commerce in pharmaceuticals and the persistent threat of counterfeit drugs, technologies like blockchain will become even more critical for ensuring end-to-end traceability, authenticity, and supply chain integrity. Smart packaging technologies, for example, will provide invaluable insights into inventory levels, item status, and location, further enhancing security and operational efficiency.
Sustainability as a Core Tenet
Sustainable procurement will move beyond a niche concern to a core tenet of pharmaceutical operations. Organizations will increasingly prioritize suppliers with strong ESG credentials, focusing on reducing environmental impact, promoting social responsibility, and contributing to a circular economy.
Strategic Partnerships and Collaboration
The trend towards deeper, more collaborative partnerships with suppliers will continue, moving away from purely transactional relationships. This fosters shared innovation, resilience, and a more robust ecosystem for delivering patient care.
Key Takeaways
- Holistic Approach: Pharmaceutical procurement is a complex, strategic function requiring expertise across pharmaceutical, managerial, legal, and financial domains.
- Regulatory Imperative: Strict adherence to regulatory frameworks (e.g., FDA, EMA, GMP) is non-negotiable and directly impacts product quality, patient safety, and market access.
- Technology as an Enabler: Digitalization, AI, machine learning, and blockchain are transforming procurement, enhancing efficiency, transparency, and risk mitigation.
- Strategic Sourcing and Supplier Relationships: Moving beyond transactional buying to forge long-term, collaborative partnerships is crucial for resilience and value creation.
- Value Over Cost: The shift towards value-based procurement prioritizes patient outcomes and total lifecycle cost over the lowest upfront price.
- Risk Management is Paramount: Proactive identification, assessment, and mitigation of risks across the supply chain are essential for continuity and patient safety.
- Ethical and Sustainable Practices: Integrating ethical sourcing and sustainable initiatives is increasingly vital for corporate responsibility and long-term viability.
- Drug Patents and Market Dynamics: Understanding drug patent expiration and leveraging generic opportunities, often with the help of tools like DrugPatentWatch, is critical for cost management and expanding patient access.
FAQs
- How do drug patents specifically influence the cost of pharmaceuticals in procurement?Drug patents grant pharmaceutical companies a temporary monopoly, typically 20 years, allowing them to set higher prices for innovative drugs to recoup significant research and development investments. This directly impacts procurement costs during the patent-protected period. Once the patent expires, generic manufacturers can produce bioequivalent versions at significantly lower prices, introducing competition and driving down costs, offering procurement teams a crucial opportunity for savings.
- What is value-based procurement, and how does it differ from traditional cost-focused procurement in the pharmaceutical sector?Value-based procurement shifts the focus from merely acquiring drugs at the lowest possible price to considering the total value a product delivers, encompassing patient outcomes, clinical effectiveness, and long-term cost savings (e.g., reduced hospital stays, fewer side effects). Traditional cost-focused procurement primarily prioritizes upfront acquisition costs. Value-based approaches encourage suppliers to offer solutions that optimize patient health and system efficiency, not just a cheap price tag.
- How is blockchain technology specifically being used to enhance transparency and security in pharmaceutical supply chains?Blockchain creates an immutable, decentralized ledger that securely records every transaction and movement of a pharmaceutical product from its raw material origin to the patient. This real-time, tamper-proof record enhances transparency by allowing all authorized stakeholders to verify a product’s authenticity and journey, significantly reducing the risk of counterfeiting, diversion, and product tampering. Smart contracts on the blockchain can also automate quality checks and regulatory compliance processes.
- What are the primary ethical considerations procurement professionals must address when sourcing pharmaceutical products globally?Globally, ethical considerations include ensuring fair labor practices in supplier facilities, preventing the use of child labor or exploitative conditions, combating the trade in counterfeit medicines, and ensuring responsible sourcing of raw materials (e.g., avoiding conflict minerals or unsustainably harvested natural ingredients). Procurement professionals must conduct thorough due diligence and build relationships with suppliers committed to ethical conduct and transparency, often adhering to principles set by organizations like the Pharmaceutical Supply Chain Initiative (PSCI).
- How can pharmaceutical companies leverage data analytics and AI to better forecast demand and mitigate supply chain disruptions?Data analytics and AI can analyze vast historical data sets, including sales trends, seasonal patterns, geopolitical events, and even real-time news feeds, to develop highly accurate demand forecasts. AI algorithms can identify subtle patterns and predict potential disruptions (e.g., from weather, political instability, or supplier performance issues), allowing procurement teams to proactively adjust inventory levels, diversify sourcing, or implement contingency plans. This shifts procurement from a reactive to a highly predictive and proactive function.
Cited Available Information
- Viseven. “Pharma Procurement Strategy: Driving Efficiency & Innovation.” Accessed July 10, 2025.
- MavenVista. “Pharma Procurement Compliance: How to Avoid Costly Regulatory Penalties.” Accessed July 10, 2025.
- Number Analytics. “Optimizing Pharmaceutical Supply Chains.” Accessed July 10, 2025.
- Buy Made Easy. “The digitalization of procurement in the pharmaceutical sector.” Accessed July 10, 2025.
- GMP SOP. “What is quality risk management in pharmaceutical?” Accessed July 10, 2025.
- Roche. “Procurement Risk Management.” Accessed July 10, 2025.
- ZIM Labs. “Ethical Considerations of Pharmaceutical Product Development.” Accessed July 10, 2025.
- Takeda Pharmaceuticals. “Sustainable Procurement.” Accessed July 10, 2025.
- GEP Blog. “Getting the Most from Your Pharmaceutical Procurement Strategy.” Accessed July 10, 2025.
- SpendEdge. “Strategic Solutions to Pharma Procurement Challenges.” Accessed July 10, 2025.
- Kearney. “Procurement trends in biopharmaceuticals.” Accessed July 10, 2025.
- PwC. “Next in pharma 2025: The future is now.” Accessed July 10, 2025.
- Supplychainstrategy.media. “2025 pharma supply chain trends: How AI and machine learning are defining the future.” Accessed July 10, 2025.
- Qualifyze. “How Procurement Can Drive Digital Innovation in Pharma.” Accessed July 10, 2025.
- WIPO. “Pat-INFORMED – The Gateway to Medicine Patent Information.” Accessed July 10, 2025.
- PitchBook. “DrugPatentWatch 2025 Company Profile: Valuation, Funding & Investors.” Accessed July 10, 2025.
- ALS.net. “Drug Patents: How Pharmaceutical IP Incentivizes Innovation and Affects Pricing.” Accessed July 10, 2025.