TADALAFIL Drug Patent Profile
✉ Email this page to a colleague
When do Tadalafil patents expire, and what generic alternatives are available?
Tadalafil is a drug marketed by Accord Hlthcare, Ajanta Pharma Ltd, Alembic, Amneal Pharms Co, Aurobindo Pharma Ltd, Austarpharma, Chartwell Rx, Cipla, Dr Reddys, Hetero Labs Ltd Iii, Lupin Ltd, Macleods Pharms Ltd, Mylan, Novitium Pharma, Prinston Inc, Qilu Pharm Hainan, Rising, Shandong, Sun Pharm, Sunshine, Teva Pharms Usa, Torrent, Umedica, Unichem, Vkt Pharma, Watson Labs Inc, and Zydus Pharms. and is included in thirty-nine NDAs.
The generic ingredient in TADALAFIL is tadalafil. There are twenty-five drug master file entries for this compound. Forty-seven suppliers are listed for this compound. Additional details are available on the tadalafil profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Tadalafil
A generic version of TADALAFIL was approved as tadalafil by TEVA PHARMS USA on May 22nd, 2018.
Summary for TADALAFIL
| US Patents: | 0 |
| Applicants: | 27 |
| NDAs: | 39 |
| Finished Product Suppliers / Packagers: | 44 |
| Raw Ingredient (Bulk) Api Vendors: | 108 |
| Clinical Trials: | 212 |
| Patent Applications: | 6,425 |
| Formulation / Manufacturing: | see details |
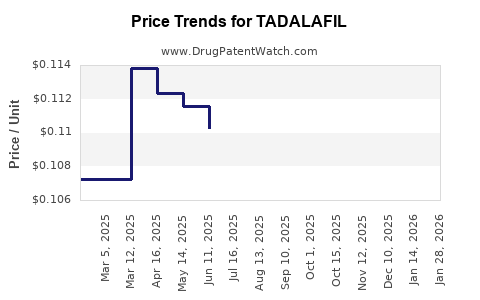
| Drug Prices: | Drug price information for TADALAFIL |
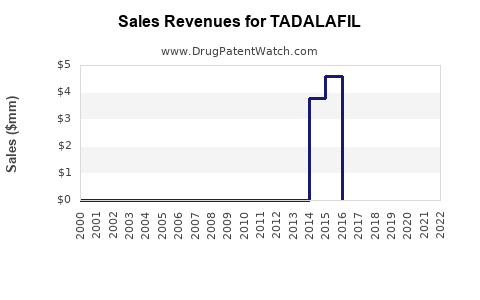
| Drug Sales Revenues: | Drug sales revenues for TADALAFIL |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for TADALAFIL |
| What excipients (inactive ingredients) are in TADALAFIL? | TADALAFIL excipients list |
| DailyMed Link: | TADALAFIL at DailyMed |
Recent Clinical Trials for TADALAFIL
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Assistance Publique - Hôpitaux de Paris | Phase 3 |
| Benha University | N/A |
| University of Arizona | Phase 2 |
Pharmacology for TADALAFIL
| Drug Class | Phosphodiesterase 5 Inhibitor |
| Mechanism of Action | Phosphodiesterase 5 Inhibitors |
Medical Subject Heading (MeSH) Categories for TADALAFIL
Anatomical Therapeutic Chemical (ATC) Classes for TADALAFIL
US Patents and Regulatory Information for TADALAFIL
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rising | TADALAFIL | tadalafil | TABLET;ORAL | 206956-003 | Apr 29, 2019 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Vkt Pharma | TADALAFIL | tadalafil | TABLET;ORAL | 215556-002 | Nov 4, 2021 | AB1 | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Dr Reddys | TADALAFIL | tadalafil | TABLET;ORAL | 210145-001 | Feb 5, 2019 | AB2 | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Cipla | TADALAFIL | tadalafil | TABLET;ORAL | 209539-001 | Mar 26, 2019 | AB1 | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Teva Pharms Usa | TADALAFIL | tadalafil | TABLET;ORAL | 090141-004 | May 22, 2018 | AB1 | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Lupin Ltd | TADALAFIL | tadalafil | TABLET;ORAL | 210567-003 | Mar 26, 2019 | AB1 | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Zydus Pharms | TADALAFIL | tadalafil | TABLET;ORAL | 206693-004 | Mar 26, 2019 | AB1 | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for TADALAFIL
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Viatris Limited | Talmanco (previously Tadalafil Generics) | tadalafil | EMEA/H/C/004297 Talmanco is indicated in adults for the treatment of pulmonary arterial hypertension (PAH) classified as WHO functional class II and III, to improve exercise capacity. Efficacy has been shown in idiopathic PAH (IPAH) and in PAH related to collagen vascular disease. |
Authorised | yes | no | no | 2017-01-09 | |
| Eli Lilly Nederland B.V. | Tadalafil Lilly | tadalafil | EMEA/H/C/004666 Treatment of erectile dysfunction in adult males.In order for tadalafil to be effective, sexual stimulation is required.Tadalafil Lilly is not indicated for use by women.Treatment of the signs and symptoms of benign prostatic hyperplasia in adult males. |
Authorised | no | no | no | 2017-03-22 | |
| Eli Lilly Nederland B.V. | Adcirca (previously Tadalafil Lilly) | tadalafil | EMEA/H/C/001021 AdultsTreatment of pulmonary arterial hypertension (PAH) classified as WHO functional class II and III, to improve exercise capacity (see section 5.1).Efficacy has been shown in idiopathic PAH (IPAH) and in PAH related to collagen vascular disease.Paediatric populationTreatment of paediatric patients aged 2 years and above with pulmonary arterial hypertension (PAH) classified as WHO functional class II and III. |
Authorised | no | no | no | 2008-10-01 | |
| Eli Lilly Nederland B.V. | Cialis | tadalafil | EMEA/H/C/000436 Treatment of erectile dysfunction.In order for tadalafil to be effective, sexual stimulation is required.Cialis is not indicated for use by women. |
Authorised | no | no | no | 2002-11-12 | |
| Mylan Pharmaceuticals Limited | Tadalafil Mylan | tadalafil | EMEA/H/C/003787 Treatment of erectile dysfunction in adult males.In order for tadalafil to be effective, sexual stimulation is required.Tadalafil Mylan is not indicated for use by women. |
Authorised | yes | no | no | 2014-11-21 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |


