RESTASIS Drug Patent Profile
✉ Email this page to a colleague
When do Restasis patents expire, and when can generic versions of Restasis launch?
Restasis is a drug marketed by Abbvie and is included in one NDA. There are ten patents protecting this drug.
The generic ingredient in RESTASIS is cyclosporine. There are eighteen drug master file entries for this compound. Eighteen suppliers are listed for this compound. Additional details are available on the cyclosporine profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Restasis
A generic version of RESTASIS was approved as cyclosporine by HIKMA on October 29th, 1999.
Summary for RESTASIS
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 4 |
| Raw Ingredient (Bulk) Api Vendors: | 122 |
| Clinical Trials: | 51 |
| Patent Applications: | 4,528 |
| Formulation / Manufacturing: | see details |
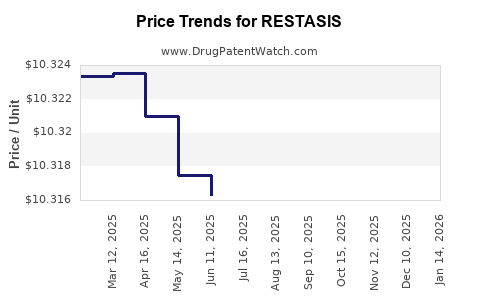
| Drug Prices: | Drug price information for RESTASIS |
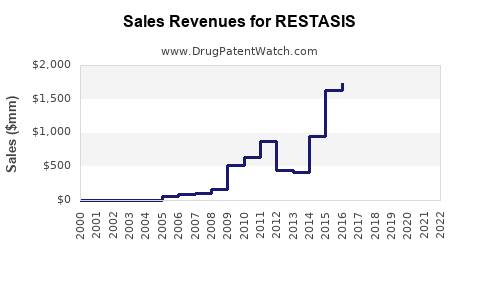
| Drug Sales Revenues: | Drug sales revenues for RESTASIS |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for RESTASIS |
| What excipients (inactive ingredients) are in RESTASIS? | RESTASIS excipients list |
| DailyMed Link: | RESTASIS at DailyMed |
Recent Clinical Trials for RESTASIS
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| OphRx Ltd. | Early Phase 1 |
| Boston Sight | Phase 1/Phase 2 |
| Allergan | Phase 1/Phase 2 |
Pharmacology for RESTASIS
| Drug Class | Calcineurin Inhibitor Immunosuppressant |
| Mechanism of Action | Calcineurin Inhibitors Cytochrome P450 3A4 Inhibitors P-Glycoprotein Inhibitors |
US Patents and Regulatory Information for RESTASIS
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbvie | RESTASIS | cyclosporine | EMULSION;OPHTHALMIC | 050790-001 | Dec 23, 2002 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Abbvie | RESTASIS MULTIDOSE | cyclosporine | EMULSION;OPHTHALMIC | 050790-002 | Oct 27, 2016 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Abbvie | RESTASIS MULTIDOSE | cyclosporine | EMULSION;OPHTHALMIC | 050790-002 | Oct 27, 2016 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Abbvie | RESTASIS MULTIDOSE | cyclosporine | EMULSION;OPHTHALMIC | 050790-002 | Oct 27, 2016 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for RESTASIS
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Abbvie | RESTASIS | cyclosporine | EMULSION;OPHTHALMIC | 050790-001 | Dec 23, 2002 | ⤷ Sign Up | ⤷ Sign Up |
| Abbvie | RESTASIS | cyclosporine | EMULSION;OPHTHALMIC | 050790-001 | Dec 23, 2002 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for RESTASIS
See the table below for patents covering RESTASIS around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Germany | 69522104 | ⤷ Sign Up | |
| European Patent Office | 0759773 | EMULSION SPECIFIQUE DE LA GLANDE LACRYMALE POUR APPLICATION LOCALE SUR LES TISSUS DE L' IL (LACRIMAL GLAND SPECIFIC EMULSIONS FOR TOPICAL APPLICATION TO OCULAR TISSUE) | ⤷ Sign Up |
| Spain | 2012116 | METODO PARA LA PRODUCCION DE UNA COMPOSICION OFTALMICA A BASE DE CICLOSPORINA DE USO POR VIA LOCAL. (OCULAR CYCLOSPORIN COMPOSITION.) | ⤷ Sign Up |
| Greece | 1000558 | ΜΕΘΟΔΟΣ ΠΑΡΑΣΚΕΥΗΣ ΟΦΘΑΛΜΙΚΗΣ ΣΥΝΘΕΣΕΩΣ ΚΥΚΛΟΣΠΟΡΙΝΗΣ. (OPHTHALMOLOGICAL CYCLOSPORINE COMPOSITION AND PREPARATION METHOD) | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for RESTASIS
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2049079 | LUC00006 | Luxembourg | ⤷ Sign Up | PRODUCT NAME: CYCLOSPORINE (GOUTTES OCULAIRES SOUS FORME D'EMULSION); AUTHORISATION NUMBER AND DATE: EU/1/15/990 20150323 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


