METOPROLOL Drug Patent Profile
✉ Email this page to a colleague
When do Metoprolol patents expire, and when can generic versions of Metoprolol launch?
Metoprolol is a drug marketed by Accord Hlthcare, Actavis Elizabeth, Actavis Labs Fl Inc, Alkem Labs Ltd, Cipla, Dr Reddys Labs Ltd, Granules, Hetero Labs Ltd Iii, Ipca Labs Ltd, Lupin, Mylan Pharms Inc, Nesher Pharms, Novast Labs, Pharmadax Inc, Prinston Inc, Reddys, Sandoz, Sunshine, Visum Pharm, Wockhardt Bio Ag, Yichang Humanwell, Zhejiang Jutai Pharm, Zydus Pharms, Am Regent, Baxter Hlthcare Corp, Fresenius Kabi Usa, Gland, Hikma, Hikma Farmaceutica, Hospira, Luitpold, Onesource Specialty, Watson Labs, Alembic Pharms Ltd, Apothecon, Aurobindo Pharma, Chartwell Rx, Heritage Pharma, Mylan, Pharmobedient, Purepac Pharm, Renata, Rubicon Research, Sciegen Pharms, Sun Pharm Inds Inc, Sun Pharm Industries, Teva, Teva Pharms, Alembic, Senores Pharms, and Sun Pharm Inds. and is included in sixty-five NDAs.
The generic ingredient in METOPROLOL is hydrochlorothiazide; metoprolol tartrate. There are thirty-two drug master file entries for this compound. Five suppliers are listed for this compound. Additional details are available on the hydrochlorothiazide; metoprolol tartrate profile page.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for METOPROLOL?
- What are the global sales for METOPROLOL?
- What is Average Wholesale Price for METOPROLOL?
Summary for METOPROLOL
| US Patents: | 0 |
| Applicants: | 51 |
| NDAs: | 65 |
| Drug Prices: | Drug price information for METOPROLOL |
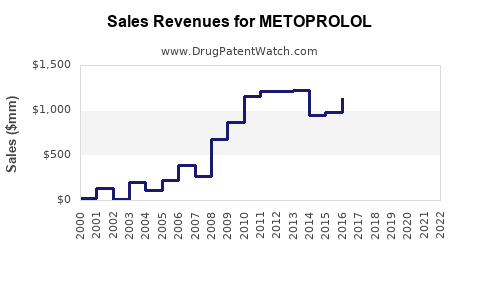
| Drug Sales Revenues: | Drug sales revenues for METOPROLOL |
| DailyMed Link: | METOPROLOL at DailyMed |
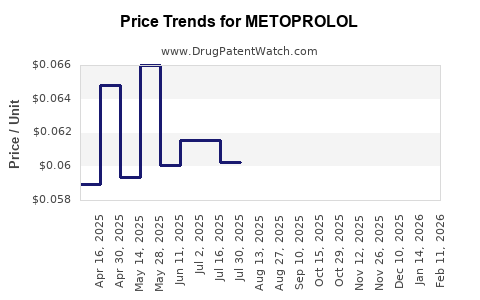
See drug prices for METOPROLOL
US Patents and Regulatory Information for METOPROLOL
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visum Pharm | METOPROLOL SUCCINATE | metoprolol succinate | TABLET, EXTENDED RELEASE;ORAL | 207206-003 | Dec 19, 2018 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Watson Labs | METOPROLOL TARTRATE | metoprolol tartrate | INJECTABLE;INJECTION | 074032-001 | Dec 21, 1993 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Lupin | METOPROLOL SUCCINATE | metoprolol succinate | TABLET, EXTENDED RELEASE;ORAL | 209272-004 | Aug 15, 2023 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Hetero Labs Ltd Iii | METOPROLOL SUCCINATE | metoprolol succinate | TABLET, EXTENDED RELEASE;ORAL | 205541-003 | Nov 6, 2020 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
