Last updated: October 15, 2025
Introduction
Lactulose, a synthetic disaccharide used primarily as a laxative and in managing hepatic encephalopathy, has sustained a significant presence within the pharmaceutical landscape. Its unique mechanism, coupled with expanding indications, positions lactulose as a critical asset in gastrointestinal and neuropsychiatric therapy. This analysis explores the current market landscape, growth drivers, competitive dynamics, regulatory factors, and financial outlook shaping lactulose's future trajectory.
Market Overview and Growth Drivers
Global Market Size and Trends
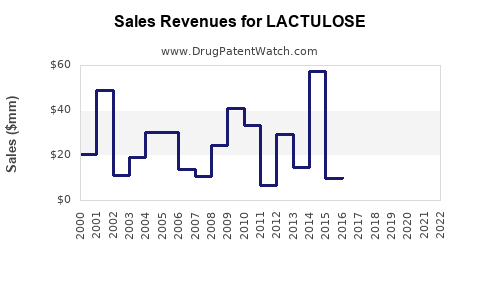
The global lactulose market was valued at approximately USD 350 million in 2022 and is projected to grow at a Compound Annual Growth Rate (CAGR) of 4-6% over the next five years [1]. The expansion is driven by rising prevalence of liver diseases, increasing geriatric populations, and growing awareness of bowel health management.
Key Indications and Expanding Usage
Lactulose's primary indications include:
- Chronic Constipation: A long-standing use, enhanced by increasing consumer health consciousness.
- Hepatic Encephalopathy: The dominant therapeutic usage, where lactulose reduces ammonia absorption, thereby improving neurocognitive functions in patients with cirrhosis.
Emerging uses in bowel cleansing procedures and diverticulitis management further diversify its application base.
Prevalence of Target Conditions
The global burden of liver cirrhosis and chronic constipation substantially influences demand. The World Health Organization estimates over 1.5 billion individuals globally suffer from chronic constipation, and liver cirrhosis accounts for approximately 1 million deaths annually [2], fostering a continual need for effective treatments like lactulose.
Demographic and Lifestyle Factors
An aging global population and lifestyle factors such as high-fat diets and sedentarism fuel gastrointestinal disorders, reinforcing demand projections. Additionally, increasing healthcare awareness facilitates earlier intervention, boosting market size.
Competitive Landscape and Market Players
Key Manufacturers
Leading companies include:
- Ursapharm (Germany)
- Fresenius Kabi (Germany)
- Mylan (USA)
- LGM Pharma (India)
- Hovid (Malaysia)
These entities hold dominant shares, leveraging manufacturing expertise, established distribution channels, and regulatory approvals.
Generic vs. Branded Formulations
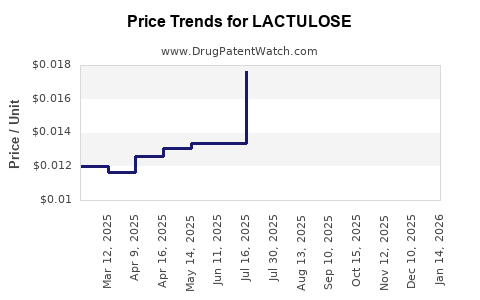
The market is predominantly composed of generics, making competitive pricing a decisive factor. Patent expirations of formulations have led to a proliferation of low-cost alternatives, intensifying price competition.
Research and Development
While current formulations are well-established, ongoing R&D focuses on novel delivery mechanisms to enhance patient compliance, such as sachets, liquid formulations with improved taste, and combination therapies.
Regulatory and Patent Considerations
Regulatory Pathways
Lactulose is generally recognized as safe (GRAS) and has widespread regulatory approval, facilitating rapid market access. However, formulation-specific patents can affect market exclusivity periods.
Patent Expirations and Market Entry Barriers
The expiration of patents on key formulations has opened avenues for competitors but also increased market penetration challenges for biosimilar entrants. Regulatory hurdles, especially for new delivery systems, remain a barrier to innovation-driven market share growth.
Pricing Trends and Reimbursement Landscape
Price margins remain compressed, especially in mature markets like the U.S. and EU, due to price regulation and competitive generic offerings. Reimbursement policies generally favor cost-effective therapies, emphasizing the importance of pricing strategies for market sustainability.
Future Financial Trajectory and Market Drivers
Projected Growth Factors
- Increasing prevalence of liver diseases: Growing burden of hepatitis, alcohol-related cirrhosis, and non-alcoholic fatty liver disease (NAFLD) predicts sustained demand.
- Healthcare infrastructure expansion: Emerging markets in Asia-Pacific, Africa, and Latin America are investing in healthcare, increasing access and utilization.
- Ethnic and demographic shifts: Aging populations in Europe and North America position lactulose as a staple in chronic disease management.
Market Challenges
- Pricing pressures: Economies of scale and competition may exert downward price forces.
- Competition from alternative therapies: Newer neurotherapy agents and laxatives could challenge lactulose's market share.
- Formulation challenges: Improving palatability and patient adherence remains an ongoing concern.
Financial Outlook
Based on historical growth and current demand dynamics, annual revenues are expected to reach USD 500-600 million by 2028, with select markets offering higher margins due to localized pricing and reimbursement policies.
Strategic Opportunities
- Product innovation: Developing more palatable formulations and combination therapies can expand usage.
- Market penetration: Targeting underserved emerging markets presents significant growth prospects.
- Regulatory engagement: Streamlining approval processes for new indications will accelerate revenue streams.
Conclusion
Lactulose's market remains robust, driven by high-prevalence indications and aging populations. The financial trajectory indicates steady growth, contingent on effective competitive strategies, regulatory navigation, and innovation adoption. Companies that can optimize supply chains, tailoring formulations to diverse markets while managing pricing pressures, stand to capitalize on the evolving landscape.
Key Takeaways
- The global lactulose market is projected to grow at a CAGR of 4-6%, driven mainly by gastrointestinal and hepatic indications.
- Patent expirations and the rise of generics have intensified price competition, demanding strategic positioning from manufacturers.
- Emerging markets and aging demographic trends present significant revenue growth avenues.
- Innovation in formulation and targeted marketing are critical to differentiate and expand share.
- Regulatory stability and reimbursement policies profoundly influence market stability and profit margins.
FAQs
1. What are the main therapeutic indications for lactulose?
Lactulose is primarily used for chronic constipation and hepatic encephalopathy. Emerging applications include bowel cleansing and diverticulitis management.
2. How does patent expiration impact lactulose market dynamics?
Patent expiries facilitate generic entry, lowering prices and increasing accessibility. However, they also challenge brand loyalty and profit margins for originators.
3. What challenges do companies face in expanding lactulose markets?
Pricing competition, formulation preferences, regulatory hurdles, and competition from newer therapies pose significant challenges.
4. Which regions are expected to drive future lactulose demand?
Emerging markets in Asia-Pacific, Africa, and Latin America, along with aging populations in North America and Europe, will significantly influence future demand.
5. How can manufacturers sustain profitability amid price pressures?
Investing in formulation innovation, entering new indications, expanding regional presence, and optimizing supply chain efficiency are vital strategies.
References
[1] MarketDataForecast, "Lactulose Market Forecast," 2022.
[2] WHO, "Global Burden of Liver Diseases," 2021.