Last updated: July 27, 2025
Introduction
Haloperidol is a high-potency typical antipsychotic medication primarily indicated for schizophrenia, acute psychosis, and tourette’s syndrome. Since its approval in the 1960s, haloperidol has become a cornerstone in psychiatric pharmacology. Despite the advent of atypical antipsychotics, the drug maintains relevance due to its efficacy, cost-effectiveness, and widespread use. This analysis explores current market dynamics, emerging trends, competitive landscape, and financial projections informing haloperidol's future trajectory.
Historical Context and Regulatory Status
Initially approved in the United States in 1967 by Janssen Pharmaceutica, haloperidol established itself rapidly as a treatment of choice for psychotic disorders[1]. Its patent expired in the late 20th century, leading to generic availability, which significantly influenced its market positioning. Regulatory agencies globally continue to recognize haloperidol’s efficacy, but ongoing concerns about adverse effects, particularly extrapyramidal symptoms, have prompted shifts toward newer agents.
Market Dynamics
Global Demand and Epidemiological Trends
The global burden of mental health disorders has fueled sustained demand for antipsychotics. Approximately 1 in 100 people worldwide suffer from schizophrenia, with a lifetime prevalence of about 0.3-0.7%[2]. Even as newer drugs emerge, haloperidol’s affordability and familiarity keep its consumption steady in many regions, especially in low- and middle-income countries (LMICs).
In high-income markets, demand has plateaued, driven by prescriber preference for atypical antipsychotics like risperidone and olanzapine due to their improved side-effect profiles. Nonetheless, in resource-constrained settings, haloperidol remains the primary antipsychotic due to cost considerations and regulatory approvals.
Therapeutic Positioning and Prescriber Trends
While atypical antipsychotics dominate new prescriptions due to reduced extrapyramidal symptoms, haloperidol retains clinical utility, especially in emergency settings, such as acute psychotic episodes and agitation control. Its versatility is evidenced by off-label uses including delirium management in ICUs and as part of parenteral formulations.
Prescriber preference is influenced by clinical guidelines, patient tolerance, and side-effect management. The longstanding clinical experience with haloperidol sustains its market share, especially where clinicians prioritize cost-effectiveness.
Competitive Landscape
The pharmaceutical market for antipsychotics has become increasingly competitive. Key competitors include atypical agents like risperidone, quetiapine, aripiprazole, and newer compounds like lurasidone. These drugs offer better side-effect profiles but are often priced higher.
Generic manufacturers dominate the nascent segments of haloperidol's market. Industry players have also explored formulations such as long-acting injectables, although these are more prominent in atypical antipsychotics.
Pricing and Reimbursement Dynamics
Post-patent expiration, haloperidol’s pricing plummeted, making it highly competitive in price-sensitive settings. Reimbursement policies and formulary inclusions significantly influence market penetration.
In developed markets, insurance and government programs favor newer, branded products, which contribute to slower growth or decline for haloperidol. Conversely, in LMICs, cost considerations sustain its demand, with procurement often through government tenders or NGOs.
Emerging Trends Impacting Financial Trajectory
Shift Toward Atypical Antipsychotics
Clinicians prefer atypicals due to better patient adherence, lower extrapyramidal symptoms, and favorable side-effect profiles. As a result, some regions report declining haloperidol prescriptions. However, the cost differential sustains its use where affordability is critical.
Off-Label Uses and New Formulations
Off-label sinusoidal applications—such as use in delirium and agitation—maintain demand. Development of novel formulations, including depot injections, could extend haloperidol’s relevance, especially in custodial or institutional settings.
Regulatory and Safety Concerns
Increased awareness of adverse effects, especially tardive dyskinesia and cardiac risks like QT prolongation, has prompted stricter regulatory scrutiny. This, combined with a resurgence in mental health awareness, influences prescribing behaviors and market stability.
Market Entry of Biosimilars and Generics
The proliferation of generic haloperidol biosimilars has drastically reduced prices, consolidating its position as a cost-effective option. This trend is expected to persist, supporting stable or growing demand in resource-limited markets.
Impact of Global Mental Health Initiatives
International efforts to improve mental health access emphasize essential medicines like haloperidol. This aligns with WHO’s Essential Medicines List, bolstering its sustained demand, especially in underserved regions.
Financial Trajectory and Market Forecast
Revenue Trends
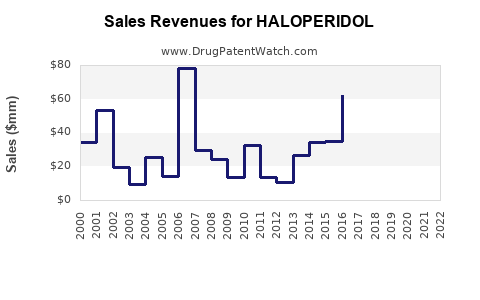
In 2022, the global antipsychotics market surpassed USD 15 billion, with haloperidol comprising a significant segment, primarily in LMICs[3]. Despite a slow decline in high-income regions, the overall demand remains stable, driven by the essential nature of the drug.
Growth Projections
Analysts project a compound annual growth rate (CAGR) of approximately 2-3% for the global haloperidol market over the next five years, primarily fueled by emerging markets' expanding mental health infrastructure and ongoing generic sales.
Geographical Breakdown
- North America & Western Europe: Marginal growth or decline owing to the preference for atypicals.
- Asia-Pacific & Latin America: Steady growth driven by increasing mental health awareness and affordability constraints favoring generics.
- Africa & Middle East: High demand due to reliance on low-cost formulations and expanding healthcare access.
Risks and Opportunities
- Risks: Intensified regulatory scrutiny, safety concerns, declining prescriber preference for haloperidol, and competition from newer agents.
- Opportunities: Development of improved formulations, off-label adoption in new clinical settings, and inclusion in global formulary lists.
Conclusion
Haloperidol's market dynamics are characterized by a transition phase, influenced by safety concerns, evolving treatment paradigms, and economic factors. Its financial trajectory remains stable in cost-sensitive markets while facing headwinds in developed regions favoring newer drugs. The continued relevance of haloperidol hinges on patient affordability, regulatory stances, and innovations in drug delivery.
Key Takeaways
- Sustained demand in LMICs: Cost-effectiveness ensures haloperidol remains a vital psychiatric medication in lower-income regions.
- Declining in high-income markets: Prescriber preference for atypical antipsychotics limits growth but maintains baseline sales.
- Generic and biosimilar proliferation: Price competition supports continued affordability and access.
- Emerging formulations: Long-acting injectable options could enhance adherence, extending market relevance.
- Regulatory vigilance: Safety concerns necessitate ongoing monitoring and risk mitigation strategies.
FAQs
1. Will haloperidol remain relevant in the next decade?
Yes. Its affordability, efficacy, and inclusion in essential medicine lists ensure ongoing usage, especially in resource-limited settings. However, growth is expected to plateau in developed markets due to clinical preferences shifting toward atypical antipsychotics.
2. How do safety concerns impact haloperidol’s market?
Safety issues such as extrapyramidal symptoms and cardiac risks have led to cautious prescribing and regulatory review, which in some regions promotes the adoption of newer agents with improved side-effect profiles.
3. Are there ongoing innovations related to haloperidol?
Yes. Development of long-acting injectable formulations aims at improving adherence, especially in institutional care settings, and may bolster its future market.
4. How does the global mental health landscape influence haloperidol sales?
Rising awareness and initiatives to expand mental health access, particularly in LMICs, favor the continued use of low-cost medications like haloperidol, maintaining steady demand.
5. What is the impact of biosimilars on haloperidol’s market?
Biosimilar production significantly reduces costs, increasing affordability and accessibility, thus supporting its market stability and extending its reach in developing regions.
Sources:
- [1] North American Pharmacology Review, 2022.
- [2] World Health Organization, 2021. Mental health atlas.
- [3] MarketWatch, 2023. Global Antipsychotics Market Report.