FLUVOXAMINE MALEATE Drug Patent Profile
✉ Email this page to a colleague
When do Fluvoxamine Maleate patents expire, and when can generic versions of Fluvoxamine Maleate launch?
Fluvoxamine Maleate is a drug marketed by Actavis Elizabeth, Bionpharma, Endo Operations, Torrent, Ani Pharms, Apotex, Chartwell Rx, Heritage Pharma, Mylan, Sun Pharm Industries, Synthon Pharms, Teva, and Upsher Smith Labs. and is included in eighteen NDAs.
The generic ingredient in FLUVOXAMINE MALEATE is fluvoxamine maleate. There are eleven drug master file entries for this compound. Twelve suppliers are listed for this compound. Additional details are available on the fluvoxamine maleate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Fluvoxamine Maleate
A generic version of FLUVOXAMINE MALEATE was approved as fluvoxamine maleate by UPSHER SMITH LABS on November 29th, 2000.
AI Research Assistant
Questions you can ask:
- What is the 5 year forecast for FLUVOXAMINE MALEATE?
- What are the global sales for FLUVOXAMINE MALEATE?
- What is Average Wholesale Price for FLUVOXAMINE MALEATE?
Summary for FLUVOXAMINE MALEATE
| US Patents: | 0 |
| Applicants: | 13 |
| NDAs: | 18 |
| Finished Product Suppliers / Packagers: | 9 |
| Raw Ingredient (Bulk) Api Vendors: | 142 |
| Clinical Trials: | 6 |
| Patent Applications: | 1,769 |
| Formulation / Manufacturing: | see details |
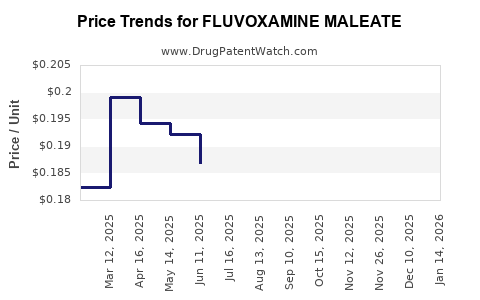
| Drug Prices: | Drug price information for FLUVOXAMINE MALEATE |
| What excipients (inactive ingredients) are in FLUVOXAMINE MALEATE? | FLUVOXAMINE MALEATE excipients list |
| DailyMed Link: | FLUVOXAMINE MALEATE at DailyMed |
Recent Clinical Trials for FLUVOXAMINE MALEATE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| The Medical Research Network | Phase 4 |
| AbbVie | Phase 4 |
| Haining Health-Coming Biotech Co., Ltd. | Phase 2 |
Pharmacology for FLUVOXAMINE MALEATE
| Drug Class | Serotonin Reuptake Inhibitor |
| Mechanism of Action | Serotonin Uptake Inhibitors |
Medical Subject Heading (MeSH) Categories for FLUVOXAMINE MALEATE
Anatomical Therapeutic Chemical (ATC) Classes for FLUVOXAMINE MALEATE
Paragraph IV (Patent) Challenges for FLUVOXAMINE MALEATE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| LUVOX CR | Extended-release Capsules | fluvoxamine maleate | 100 mg | 022033 | 1 | 2009-04-20 |
| LUVOX CR | Extended-release Capsules | fluvoxamine maleate | 150 mg | 022033 | 1 | 2009-04-13 |
US Patents and Regulatory Information for FLUVOXAMINE MALEATE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Teva | FLUVOXAMINE MALEATE | fluvoxamine maleate | TABLET;ORAL | 075893-001 | Sep 10, 2002 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Mylan | FLUVOXAMINE MALEATE | fluvoxamine maleate | TABLET;ORAL | 075950-001 | Oct 15, 2001 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Upsher Smith Labs | FLUVOXAMINE MALEATE | fluvoxamine maleate | TABLET;ORAL | 075888-003 | Nov 29, 2000 | AB | RX | No | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Upsher Smith Labs | FLUVOXAMINE MALEATE | fluvoxamine maleate | TABLET;ORAL | 075888-001 | Nov 29, 2000 | AB | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Heritage Pharma | FLUVOXAMINE MALEATE | fluvoxamine maleate | TABLET;ORAL | 075894-002 | Apr 18, 2001 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Mylan | FLUVOXAMINE MALEATE | fluvoxamine maleate | TABLET;ORAL | 075889-003 | Nov 29, 2000 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |


