Drug Price Trends for FLUVOXAMINE MALEATE
✉ Email this page to a colleague
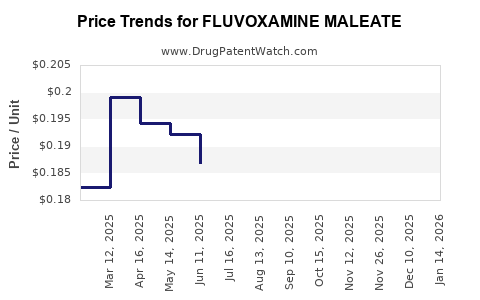
Average Pharmacy Cost for FLUVOXAMINE MALEATE
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| FLUVOXAMINE MALEATE 100 MG TAB | 60505-0166-01 | 0.19131 | EACH | 2024-11-20 |
| FLUVOXAMINE MALEATE 100 MG TAB | 69452-0289-20 | 0.19131 | EACH | 2024-11-20 |
| FLUVOXAMINE MALEATE 25 MG TAB | 00832-1670-11 | 0.15957 | EACH | 2024-11-20 |
| FLUVOXAMINE MALEATE 100 MG TAB | 62559-0160-01 | 0.19131 | EACH | 2024-11-20 |
| FLUVOXAMINE MALEATE 50 MG TAB | 69452-0288-20 | 0.15923 | EACH | 2024-11-20 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Best Wholesale Price for FLUVOXAMINE MALEATE
| Drug Name | Vendor | NDC | Count | Price ($) | Price/Unit ($) | Unit | Dates | Price Type |
|---|---|---|---|---|---|---|---|---|
| FLUVOXAMINE MALEATE 100MG TAB | AvKare, LLC | 60505-0166-01 | 100 | 53.34 | 0.53340 | EACH | 2023-08-01 - 2028-06-14 | FSS |
| FLUVOXAMINE MALEATE 100MG TAB | AvKare, LLC | 60505-0166-01 | 100 | 53.07 | 0.53070 | EACH | 2023-08-17 - 2028-06-14 | FSS |
| FLUVOXAMINE MALEATE 25MG TAB | AvKare, LLC | 60505-0164-01 | 100 | 47.74 | 0.47740 | EACH | 2023-06-15 - 2028-06-14 | FSS |
| FLUVOXAMINE MALEATE 100MG CAP,SA | AvKare, LLC | 00228-2848-03 | 30 | 214.99 | 7.16633 | EACH | 2023-06-15 - 2028-06-14 | FSS |
| FLUVOXAMINE MALEATE 25MG TAB | AvKare, LLC | 60505-0164-01 | 100 | 44.22 | 0.44220 | EACH | 2023-08-01 - 2028-06-14 | FSS |
| >Drug Name | >Vendor | >NDC | >Count | >Price ($) | >Price/Unit ($) | >Unit | >Dates | >Price Type |


