DEFERASIROX Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Deferasirox, and what generic alternatives are available?
Deferasirox is a drug marketed by Alkem Labs Ltd, Amneal, Annora Pharma, Aurobindo Pharma, Cipla, Epic Pharma Llc, MSN, Pharmobedient, Teva Pharms Usa, Actavis Elizabeth, Alembic, Bionpharma, Glenmark Speclt, Sun Pharm, Torrent, Zydus Pharms, Aurobindo Pharma Ltd, Chartwell Rx, Jubilant Generics, Piramal, and Stevens J. and is included in thirty-five NDAs.
The generic ingredient in DEFERASIROX is deferasirox. There are twenty drug master file entries for this compound. Twenty-one suppliers are listed for this compound. Additional details are available on the deferasirox profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Deferasirox
A generic version of DEFERASIROX was approved as deferasirox by ACTAVIS ELIZABETH on January 26th, 2016.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for DEFERASIROX?
- What are the global sales for DEFERASIROX?
- What is Average Wholesale Price for DEFERASIROX?
Summary for DEFERASIROX
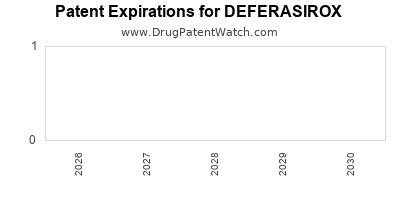
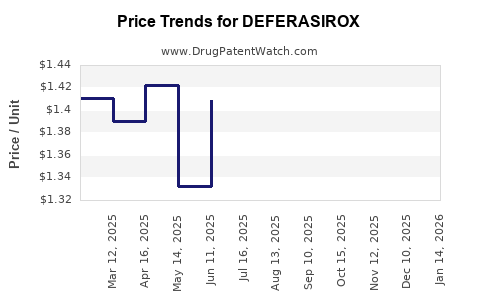
Recent Clinical Trials for DEFERASIROX
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| National Heart, Lung, and Blood Institute (NHLBI) | PHASE2 |
| University of Michigan Rogel Cancer Center | PHASE2 |
| National Institutes of Health (NIH) | PHASE2 |
Pharmacology for DEFERASIROX
| Drug Class | Iron Chelator |
| Mechanism of Action | Cytochrome P450 1A2 Inhibitors Cytochrome P450 2C8 Inhibitors Cytochrome P450 3A4 Inducers Iron Chelating Activity |
Anatomical Therapeutic Chemical (ATC) Classes for DEFERASIROX
Paragraph IV (Patent) Challenges for DEFERASIROX
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| JADENU | Tablets | deferasirox | 180 mg | 206910 | 1 | 2016-04-21 |
| JADENU | Tablets | deferasirox | 90 mg and 360 mg | 206910 | 1 | 2015-10-19 |
| EXJADE | Tablets for Suspension | deferasirox | 125 mg, 250 mg, and 500 mg | 021882 | 1 | 2011-10-28 |
US Patents and Regulatory Information for DEFERASIROX
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annora Pharma | DEFERASIROX | deferasirox | TABLET;ORAL | 214341-003 | May 14, 2021 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Teva Pharms Usa | DEFERASIROX | deferasirox | TABLET;ORAL | 209223-003 | Apr 24, 2020 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Glenmark Speclt | DEFERASIROX | deferasirox | TABLET, FOR SUSPENSION;ORAL | 209433-002 | Jan 6, 2020 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Alembic | DEFERASIROX | deferasirox | TABLET, FOR SUSPENSION;ORAL | 210060-002 | Nov 20, 2019 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Msn | DEFERASIROX | deferasirox | TABLET;ORAL | 210945-003 | Jun 16, 2020 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Sun Pharm | DEFERASIROX | deferasirox | TABLET, FOR SUSPENSION;ORAL | 209782-001 | Nov 20, 2019 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Zydus Pharms | DEFERASIROX | deferasirox | TABLET, FOR SUSPENSION;ORAL | 208882-003 | May 5, 2020 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for DEFERASIROX
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Mylan Pharmaceuticals Limited | Deferasirox Mylan | deferasirox | EMEA/H/C/005014Deferasirox Mylan is indicated forthe treatment of chronic iron overload due to frequent blood transfusions (≥7 ml/kg/month of packed red blood cells) in patients with beta thalassaemia major aged 6 years and olderthe treatment of chronic iron overload due to blood transfusions when deferoxamine therapy is contraindicated or inadequate in the following patient groups:in paediatric patients with beta thalassaemia major with iron overload due to frequent blood transfusions (≥7 ml/kg/month of packed red blood cells) aged 2 to 5 years,in adult and paediatric patients with beta thalassaemia major with iron overload due to infrequent blood transfusions ( | Authorised | yes | no | no | 2019-09-26 | |
| Novartis Europharm Limited | Exjade | deferasirox | EMEA/H/C/000670Exjade is indicated for the treatment of chronic iron overload due to frequent blood transfusions (≥ 7 ml/kg/month of packed red blood cells) in patients with beta thalassaemia major aged six years and older.Exjade is also indicated for the treatment of chronic iron overload due to blood transfusions when deferoxamine therapy is contraindicated or inadequate in the following patient groups:in patients with beta thalassaemia major with iron overload due to frequent blood transfusions (≥ 7 ml/kg/month of packed red blood cells) aged two to five years;in patients with beta thalassaemia major with iron overload due to infrequent blood transfusions (< 7 ml/kg/month of packed red blood cells) aged two years and older;in patients with other anaemias aged two years and older.Exjade is also indicated for the treatment of chronic iron overload requiring chelation therapy when deferoxamine therapy is contraindicated or inadequate in patients with non-transfusion-dependent thalassaemia syndromes aged 10 years and older. | Authorised | no | no | no | 2006-08-28 | |
| Accord Healthcare S.L.U. | Deferasirox Accord | deferasirox | EMEA/H/C/005156Deferasirox Accord is indicated for the treatment of chronic iron overload due to frequent blood transfusions (≥7 ml/kg/month of packed red blood cells) in patients with beta thalassaemia major aged 6 years and older.Deferasirox Accord is also indicated for the treatment of chronic iron overload due to blood transfusions when deferoxamine therapy is contraindicated or inadequate in the following patient groups:in paediatric patients with beta thalassaemia major with iron overload due to frequent blood transfusions (≥7 ml/kg/month of packed red blood cells) aged 2 to 5 years,in adult and paediatric patients with beta thalassaemia major with iron overload due to infrequent blood transfusions ( | Authorised | yes | no | no | 2020-01-09 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
Market Dynamics and Financial Trajectory for Deferasirox: An In-Depth Analysis
More… ↓
