CHLORDIAZEPOXIDE Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Chlordiazepoxide, and what generic alternatives are available?
Chlordiazepoxide is a drug marketed by Anda Repository, Chartwell Rx, Heritage Pharma, Micro Labs, Mylan Pharms Inc, Usl Pharma, Ascot, Barr, Ferrante, Halsey, Impax Labs, Ivax Sub Teva Pharms, Lederle, Mast Mm, Mylan, Parke Davis, Pioneer Pharms, Purepac Pharm, Roxane, Superpharm, Teva, Upsher Smith Labs, Vangard, Watson Labs, West Ward, Alembic, Alkem Labs Ltd, Amneal, Aurobindo Pharma Ltd, Corepharma, Dr Reddys, Misemer, Nuvo Pharms Inc, Teva Pharms Usa, and Torrent. and is included in eighty-six NDAs.
The generic ingredient in CHLORDIAZEPOXIDE is chlordiazepoxide hydrochloride; clidinium bromide. There are nine drug master file entries for this compound. Fourteen suppliers are listed for this compound. Additional details are available on the chlordiazepoxide hydrochloride; clidinium bromide profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Chlordiazepoxide
A generic version of CHLORDIAZEPOXIDE was approved as chlordiazepoxide hydrochloride; clidinium bromide by NUVO PHARMS INC on July 7th, 2020.
Summary for CHLORDIAZEPOXIDE
| US Patents: | 0 |
| Applicants: | 35 |
| NDAs: | 86 |
| Formulation / Manufacturing: | see details |
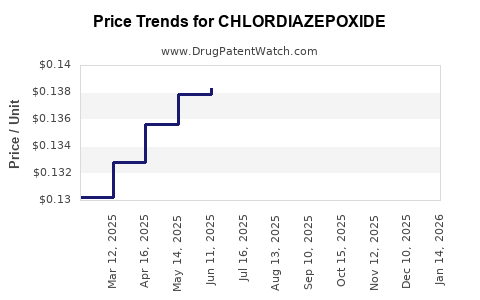
| Drug Prices: | Drug price information for CHLORDIAZEPOXIDE |
| DailyMed Link: | CHLORDIAZEPOXIDE at DailyMed |
Recent Clinical Trials for CHLORDIAZEPOXIDE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Mayo Clinic | Phase 4 |
| Oregon Health and Science University | Phase 2 |
| Taipei City Hospital | Phase 2/Phase 3 |


