AURYXIA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Auryxia, and what generic alternatives are available?
Auryxia is a drug marketed by Keryx Biopharms and is included in one NDA. There are fourteen patents protecting this drug.
This drug has one hundred and twenty-two patent family members in twenty-three countries.
The generic ingredient in AURYXIA is ferric citrate. There are twenty drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the ferric citrate profile page.
DrugPatentWatch® Generic Entry Outlook for Auryxia
There have been six patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for AURYXIA
| International Patents: | 122 |
| US Patents: | 14 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 90 |
| Clinical Trials: | 9 |
| Patent Applications: | 668 |
| Formulation / Manufacturing: | see details |
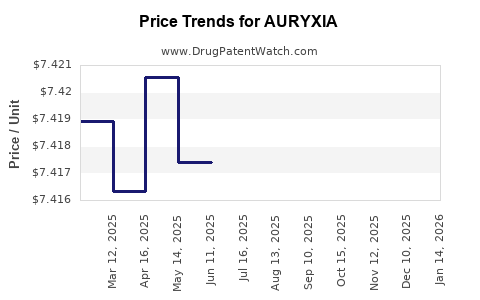
| Drug Prices: | Drug price information for AURYXIA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for AURYXIA |
| What excipients (inactive ingredients) are in AURYXIA? | AURYXIA excipients list |
| DailyMed Link: | AURYXIA at DailyMed |
Recent Clinical Trials for AURYXIA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Akebia Therapeutics | Phase 3 |
| USRC Kidney Research | Phase 3 |
| Akebia Therapeutics Inc. | Phase 4 |
Pharmacology for AURYXIA
| Drug Class | Parenteral Iron Replacement Phosphate Binder |
| Mechanism of Action | Phosphate Chelating Activity |
Anatomical Therapeutic Chemical (ATC) Classes for AURYXIA
US Patents and Regulatory Information for AURYXIA
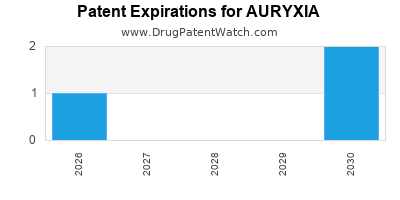
AURYXIA is protected by fourteen US patents.
Patents protecting AURYXIA
Ferric citrate dosage forms
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: CONTROL OF SERUM PHOSPHORUS LEVELS
Ferric organic compounds, uses thereof and methods of making same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pharmaceutical-grade ferric organic compounds, uses thereof and method of making same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: CONTROL OF SERUM PHOSPHOROUS LEVELS
Pharmaceutical-grade ferric organic compounds, uses thereof and method of making same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Ferric organic compounds, uses thereof and methods of making same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: CONTROL OF SERUM PHOSPHOROUS LEVELS
Ferric organic compounds, uses thereof and methods of making same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pharmaceutical-grade ferric organic compounds, uses thereof and methods of making same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Ferric organic compounds, uses thereof and methods of making same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Ferric organic compounds, uses thereof and methods of making same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: CONTROL OF SERUM PHOSPHOROUS LEVELS
Ferric organic compounds, uses thereof and methods of making same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: CONTROL OF SERUM PHOSPHOROUS LEVELS
Pharmaceutical-grade ferric organic compounds, uses thereof and methods of making same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: CONTROL OF SERUM PHOSPHOROUS LEVELS
Ferric organic compounds, uses thereof and methods of making same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: CONTROL OF SERUM PHOSPHOROUS LEVELS
Ferric citrate dosage forms
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pharmaceutical-grade ferric organic compounds, uses thereof and methods of making same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: CONTROL OF SERUM PHOSPHOROUS LEVELS
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Keryx Biopharms | AURYXIA | ferric citrate | TABLET;ORAL | 205874-001 | Sep 5, 2014 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Keryx Biopharms | AURYXIA | ferric citrate | TABLET;ORAL | 205874-001 | Sep 5, 2014 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Keryx Biopharms | AURYXIA | ferric citrate | TABLET;ORAL | 205874-001 | Sep 5, 2014 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Keryx Biopharms | AURYXIA | ferric citrate | TABLET;ORAL | 205874-001 | Sep 5, 2014 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Keryx Biopharms | AURYXIA | ferric citrate | TABLET;ORAL | 205874-001 | Sep 5, 2014 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Keryx Biopharms | AURYXIA | ferric citrate | TABLET;ORAL | 205874-001 | Sep 5, 2014 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for AURYXIA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Keryx Biopharms | AURYXIA | ferric citrate | TABLET;ORAL | 205874-001 | Sep 5, 2014 | ⤷ Try a Trial | ⤷ Try a Trial |
| Keryx Biopharms | AURYXIA | ferric citrate | TABLET;ORAL | 205874-001 | Sep 5, 2014 | ⤷ Try a Trial | ⤷ Try a Trial |
| Keryx Biopharms | AURYXIA | ferric citrate | TABLET;ORAL | 205874-001 | Sep 5, 2014 | ⤷ Try a Trial | ⤷ Try a Trial |
| Keryx Biopharms | AURYXIA | ferric citrate | TABLET;ORAL | 205874-001 | Sep 5, 2014 | ⤷ Try a Trial | ⤷ Try a Trial |
| Keryx Biopharms | AURYXIA | ferric citrate | TABLET;ORAL | 205874-001 | Sep 5, 2014 | ⤷ Try a Trial | ⤷ Try a Trial |
| Keryx Biopharms | AURYXIA | ferric citrate | TABLET;ORAL | 205874-001 | Sep 5, 2014 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for AURYXIA
See the table below for patents covering AURYXIA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 6632842 | ⤷ Try a Trial | |
| Spain | 2699447 | ⤷ Try a Trial | |
| Japan | 2017036289 | 慢性腎疾患の治療法 (METHOD OF TREATING CHRONIC KIDNEY DISEASE) | ⤷ Try a Trial |
| Taiwan | I754951 | ⤷ Try a Trial | |
| Japan | 2001506262 | ⤷ Try a Trial | |
| New Zealand | 566743 | Pharmaceutical-grade ferric organic compounds, uses thereof and methods of making same | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |


