AMLODIPINE BESYLATE Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Amlodipine Besylate, and when can generic versions of Amlodipine Besylate launch?
Amlodipine Besylate is a drug marketed by Synthon Pharms, Accord Hlthcare, Alkem, Amneal Pharms Ny, Aurobindo Pharma, Chartwell Rx, China Resources, Cipla, Corepharma, Epic Pharma Llc, Gedeon Richter Usa, Genpharm, Graviti Pharms, Hikma, Hikma Pharms, Invagen Pharms, Lupin, Mylan, Orbion Pharms, Oxford Pharms, Pharmobedient, Polygen Pharms, Puracap Pharm, Sciegen Pharms Inc, Sovereign Pharms, Strides Pharma, Sun Pharm Inds Inc, Sun Pharm Inds Ltd, Sun Pharm Industries, Sunshine, Teva, Torrent Pharms, Unichem, Watson Labs, Wockhardt, Zydus Pharms Usa, Alembic, Apotex, Dr Reddys, Neocubes Pharma, Amneal, Aurobindo Pharma Ltd, Dr Reddys Labs Inc, Heritage, Lupin Pharms, Rising, Teva Pharms, Watson Labs Inc, Hetero Labs, Macleods Pharms Ltd, Novel Labs Inc, Strides Pharma Intl, Teva Pharms Usa, Torrent, and Lupin Ltd. and is included in seventy-two NDAs.
The generic ingredient in AMLODIPINE BESYLATE is amlodipine besylate; hydrochlorothiazide; valsartan. There are fifty drug master file entries for this compound. Five suppliers are listed for this compound. Additional details are available on the amlodipine besylate; hydrochlorothiazide; valsartan profile page.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for AMLODIPINE BESYLATE?
- What are the global sales for AMLODIPINE BESYLATE?
- What is Average Wholesale Price for AMLODIPINE BESYLATE?
Summary for AMLODIPINE BESYLATE
| US Patents: | 0 |
| Applicants: | 55 |
| NDAs: | 72 |
| Finished Product Suppliers / Packagers: | 40 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 60 |
| Patent Applications: | 5,262 |
| Drug Prices: | Drug price information for AMLODIPINE BESYLATE |
| Drug Sales Revenues: | Drug sales revenues for AMLODIPINE BESYLATE |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for AMLODIPINE BESYLATE |
| What excipients (inactive ingredients) are in AMLODIPINE BESYLATE? | AMLODIPINE BESYLATE excipients list |
| DailyMed Link: | AMLODIPINE BESYLATE at DailyMed |
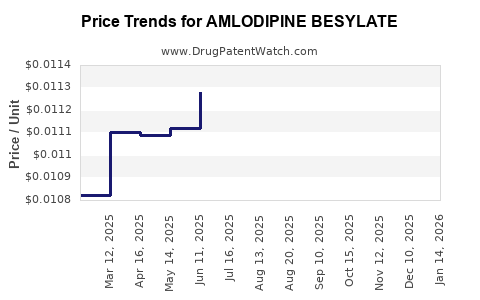
See drug prices for AMLODIPINE BESYLATE
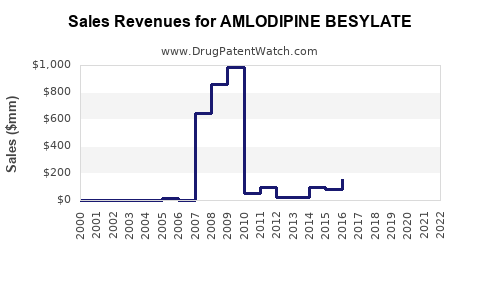
Recent Clinical Trials for AMLODIPINE BESYLATE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Third Military Medical University | PHASE4 |
| Overseas Pharmaceuticals, Ltd. | Phase 1 |
| Guangzhou Health Start Pharmaceutical Techology Co.,Ltd | Phase 1 |
Pharmacology for AMLODIPINE BESYLATE
| Drug Class | Calcium Channel Blocker Dihydropyridine Calcium Channel Blocker |
| Mechanism of Action | Calcium Channel Antagonists Cytochrome P450 3A Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for AMLODIPINE BESYLATE
US Patents and Regulatory Information for AMLODIPINE BESYLATE
Expired US Patents for AMLODIPINE BESYLATE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Synthon Pharms | AMLODIPINE BESYLATE | amlodipine besylate | TABLET, ORALLY DISINTEGRATING;ORAL | 022026-001 | Sep 27, 2007 | 6,828,339 | ⤷ Get Started Free |
| Synthon Pharms | AMLODIPINE BESYLATE | amlodipine besylate | TABLET, ORALLY DISINTEGRATING;ORAL | 022026-002 | Sep 27, 2007 | 6,828,339 | ⤷ Get Started Free |
| Synthon Pharms | AMLODIPINE BESYLATE | amlodipine besylate | TABLET, ORALLY DISINTEGRATING;ORAL | 022026-003 | Sep 27, 2007 | 6,828,339 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for AMLODIPINE BESYLATE
See the table below for patents covering AMLODIPINE BESYLATE around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 1448197 | ⤷ Get Started Free | |
| World Intellectual Property Organization (WIPO) | 03043635 | ⤷ Get Started Free | |
| Norway | 20042539 | ⤷ Get Started Free | |
| Australia | 2002343257 | ⤷ Get Started Free | |
| Argentina | 037565 | FORMAS DE SALES DE AMLODIPINA Y PROCEDIMIENTOS PARA PREPARARLAS. | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for AMLODIPINE BESYLATE
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1507558 | 2012/018 | Ireland | ⤷ Get Started Free | PRODUCT NAME: ALISKIREN OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, AMLODIPINE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF AND HYDROCHLOROTHIAZIDE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF.; NAT REGISTRATION NO/DATE: EU/1/11/730/001-060 20111122; FIRST REGISTRATION NO/DATE: SWITZERLAND 6167801-6167805 20110705 |
| 0443983 | 2007C/043 | Belgium | ⤷ Get Started Free | PRODUCT NAME: AMLODIPINE ET VALSARTAN; NATL. REGISTRATION NO/DATE: EU/1/06/370/001 20070118; FIRST REGISTRATION: CH 57771 20061222 |
| 0502314 | SPC/GB11/010 | United Kingdom | ⤷ Get Started Free | PRODUCT NAME: THE COMBINATION OF A) TELMISARTAN, OPTIONALLY IN THE FORM OF PHARMACEUTICALLY ACCEPTABLE SALTS, AND B) AMLODIPINE, OPTIONALLY IN THE FORM OF PHARMACEUTICALLY ACCEPTABLE SALTS, ESPECIALLY AMLODIPINE BESYLATE; REGISTERED: UK EU/1/10/648/001 20101007; UK EU/1/10/648/002 20101007; UK EU/1/10/648/003 20101007; UK EU/1/10/648/004 20101007; UK EU/1/10/648/005 20101007; UK EU/1/10/648/006 20101007; UK EU/1/10/648/007 20101007; UK EU/1/10/648/008 20101007; UK EU/1/10/648/009 20101007; UK EU/1/10/648/010 20101007; UK EU/1/10/648/011 20101007; UK EU/1/10/648/012 20101007; UK EU/1/10/648/013 20101007; UK EU/1/10/648/014 20101007; UK EU/1/10/648/015 20101007; UK EU/1/10/648/016 20101007; UK |
| 0678503 | C300499 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: COMBINATIE OMVATTEND ALISKIREN OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN, EN AMLODIPINE OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN; REGISTRATION NO/DATE: EU/1/11/686/001-056 20110114 |
| 0503785 | CA 2011 00026 | Denmark | ⤷ Get Started Free | PRODUCT NAME: A COMBINATION OF OLMESARTAN MEDOXOMIL, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, AND AMLODIPINE BESYLATE AND HYDROCHLOROTHIAZIDE; NAT. REG. NO/DATE: 46260-46269 (DK) 20110323; FIRST REG. NO/DATE: DE 79810.00.00 20101216 |
| 0502314 | C300478 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: TELMISARTAN, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT, EN AMLODIPINE, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT, IN HET BIJZONDER AMLODIPINEBESILAAT; REGISTRATION NO/DATE: EU/1/10/648/001-028 20101007 |
| 1003503 | 05C0048 | France | ⤷ Get Started Free | PRODUCT NAME: AMLODIPINE OU UN DE SES SELS D?ADDITION D?ACIDES PHARMACAUTIQUEMENT ACCEPTABLES/ ATORVASTATINE OU UN DE SES SELS PHARMACEUTIQUEMENT ACCEPTABLES; REGISTRATION NO/DATE IN FRANCE: NL 29929 DU 20050707; REGISTRATION NO/DATE AT EEC: NL 29929 DU 20050707 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for Amlodipine Besylate
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
