AMLODIPINE Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Amlodipine, and when can generic versions of Amlodipine launch?
Amlodipine is a drug marketed by Accord Hlthcare Inc, Ajanta Pharma Ltd, Alembic, Alkem Labs Ltd, Aurobindo Pharma, Glenmark Pharms Ltd, Hetero Labs Ltd V, Jubilant Generics, Macleods Pharms Ltd, Micro Labs, Sciegen Pharms, Teva Pharms Usa, Torrent, Zydus Pharms, Amneal, Synthon Pharms, Accord Hlthcare, Alkem, Amneal Pharms Ny, Chartwell Rx, China Resources, Cipla, Corepharma, Epic Pharma Llc, Gedeon Richter Usa, Genpharm, Graviti Pharms, Hikma, Hikma Pharms, Invagen Pharms, Lupin, Mylan, Orbion Pharms, Oxford Pharms, Pharmobedient, Puracap Labs Blu, Puracap Pharm, Sovereign Pharms, Strides Pharma, Sun Pharm Inds Inc, Sun Pharm Inds Ltd, Sun Pharm Industries, Sunshine, Teva, Torrent Pharms, Unichem, Watson Labs, Wockhardt, Zydus Pharms Usa, Apotex, Dr Reddys, Neocubes Pharma, Aurobindo Pharma Ltd, Dr Reddys Labs Inc, Heritage, Lupin Pharms, Rising, Teva Pharms, Watson Labs Inc, Hetero Labs, Novel Labs Inc, Strides Pharma Intl, and Lupin Ltd. and is included in eighty-seven NDAs.
The generic ingredient in AMLODIPINE is amlodipine besylate; hydrochlorothiazide; valsartan. There are fifty drug master file entries for this compound. Five suppliers are listed for this compound. Additional details are available on the amlodipine besylate; hydrochlorothiazide; valsartan profile page.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for AMLODIPINE?
- What are the global sales for AMLODIPINE?
- What is Average Wholesale Price for AMLODIPINE?
Summary for AMLODIPINE
| US Patents: | 0 |
| Applicants: | 63 |
| NDAs: | 87 |
| Drug Prices: | Drug price information for AMLODIPINE |
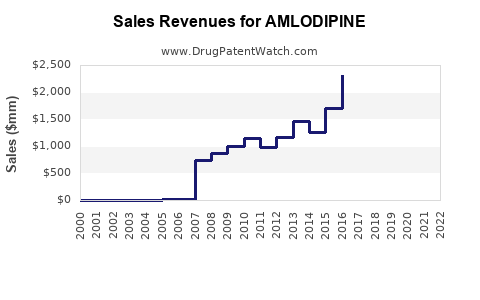
| Drug Sales Revenues: | Drug sales revenues for AMLODIPINE |
| DailyMed Link: | AMLODIPINE at DailyMed |
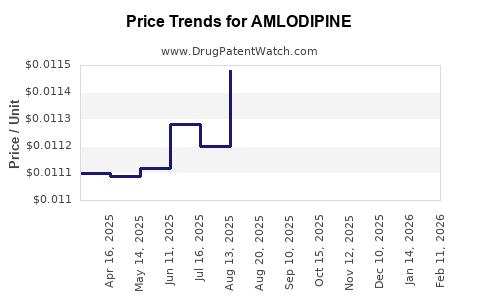
See drug prices for AMLODIPINE