Last updated: July 28, 2025
Introduction
Albuterol sulfate remains a cornerstone in respiratory therapy, primarily used for the relief of bronchospasm in conditions such as asthma, chronic obstructive pulmonary disease (COPD), and other obstructive airway diseases. Its longstanding clinical efficacy and widespread application have shaped a resilient market landscape. This analysis explores the evolving market dynamics, regulatory environment, and financial trajectory of albuterol sulfate, highlighting opportunities and challenges faced by stakeholders.
Market Overview
Albuterol sulfate, a short-acting beta-2 adrenergic agonist, is primarily administered via inhalers and nebulizers. The drug’s market has historically been robust owing to its vital role in acute care and chronic management of respiratory ailments. According to industry reports, the global inhalation therapies market was valued at approximately USD 12 billion in 2021, with albuterol-based products comprising a significant share [1].
The market is characterized by high demand in developed nations such as the US, Europe, and Japan, driven by the prevalence of respiratory diseases, while emerging regions like Asia-Pacific exhibit rapid growth due to increasing urbanization, pollution, and improved healthcare infrastructure.
Market Drivers
1. Rising Prevalence of Respiratory Diseases
The increasing incidence and prevalence of asthma and COPD, particularly in aging populations, have directly contributed to sustained demand for albuterol sulfate. The CDC estimates that over 25 million Americans suffer from asthma, with numbers rising globally, amplifying demand for bronchodilator therapies [2].
2. Advancements in Delivery Devices
Innovations such as metered-dose inhalers (MDIs), dry powder inhalers (DPIs), and nebulizers have enhanced drug delivery efficacy and patient compliance, expanding market reach. The integration of electronic inhalers with digital tracking also supports personalized treatment, further bolstering product utilization.
3. Patent Expirations and Generics
The expiration of patents for key albuterol inhalers, notably the United States patent for Ventolin (produced by GlaxoSmithKline), has precipitated a surge in generic formulations, leading to reduced prices and increased accessibility—further driving market volume.
4. Emerging Market Growth
In regions such as India and China, increasing healthcare expenditure and rising awareness about respiratory illnesses foster expanding markets for albuterol sulfate. Local manufacturers are also entering with low-cost generic versions, intensifying competition but amplifying overall volume sales.
Regulatory Environment and Patent Landscape
The regulatory framework significantly influences market dynamics. The U.S. FDA classifies albuterol inhalers as drug-device combination products requiring rigorous approval pathways. Patent cliffs—most notably, GSK’s Ventolin patent expiry in 2015—have facilitated entry of generics and biosimilars.
Regulatory approvals for generic formulations and inhaler devices are streamlined in many jurisdictions, promoting market entry but also amplifying competitive pressures. Additionally, push toward low-cost therapeutics in emerging markets influences the regulatory environment, with approvals often expedited for generics.
Market Challenges
1. Competitive Price Pressures
The surge of generic inhalers has resulted in significant price erosion, compressing profit margins for original innovator companies. This intensifies the need for product differentiation through formulation, device technology, and label expansion.
2. Market Saturation and Patent Litigation
In mature markets, saturation limits growth potential, prompting companies to seek new indications or combination therapies. Patent litigation regarding formulation and delivery device patents remains a challenge, affecting timelines for new product launches.
3. Supply Chain Disruptions
Global supply chain vulnerabilities, accentuated by geopolitical tensions and the COVID-19 pandemic, have disrupted manufacturing and distribution, impacting product availability and financial forecasts.
Financial Trajectory
Revenue Trends
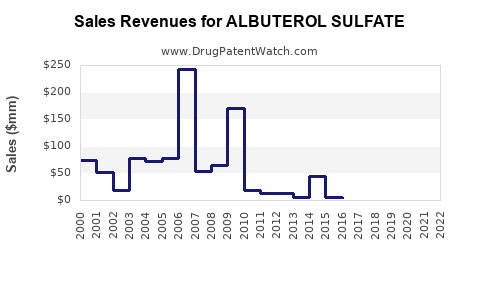
The global albuterol inhaler market has demonstrated steady growth, driven by volume increases rather than price escalation due to the proliferation of generics. In 2021, the market was valued at over USD 8 billion, with expectations to grow at a CAGR of approximately 4% through 2027 [1].
Pricing and Profitability
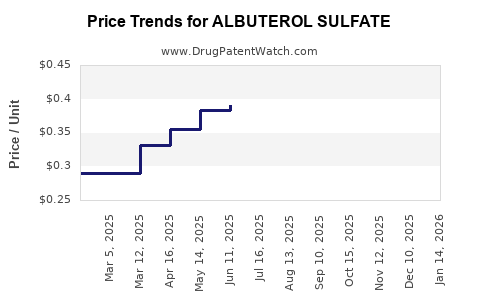
While innovator brands historically commanded premium pricing, patent expiries have led to a decline in per-unit revenue. The entry of generics around 2015 in the U.S. led to a near 50% price reduction within three years. Innovator companies are now diversifying strategies, including developing new inhaler devices, combination drugs, and expanding into new indications to sustain revenues.
Investment and R&D
R&D investments focus on improving inhaler technology, such as breath-actuated devices and digital integration. These innovations aim to enhance adherence and therapeutic outcomes, providing competitive advantages and potential for premium pricing.
Market Outlook
The outlook remains cautiously optimistic, with potential growth driven by emerging market expansion, technological innovations, and new formulation approvals. However, intensified generic competition necessitates strategic differentiation.
Future Market Trends
1. Digital and Connected Therapeutics
Integration of inhalers with smart technology supports real-time monitoring, adherence, and data collection, adding value for patients and payers. Leaders are investing heavily in digital inhaler solutions, which may command higher margins.
2. Biosimilars and Bioequivalence
The entrance of biosimilar and bioequivalent formulations is expected to increase, especially in markets with evolving biosimilar regulations. Price competition is likely to accelerate, influencing overall market revenues.
3. Personalized Medicine and Combination Therapies
Developments in personalized inhalation therapies, including combination drugs targeting multiple pathways, will influence market structure and financial trajectories.
4. Regulatory and Pricing Policies
Policy reforms favoring biosimilar adoption and cost-containment measures may further compress prices, impacting profitability but expanding access.
Key Takeaways
-
The global albuterol sulfate market remains resilient, with steady growth driven by rising respiratory disease prevalence and technological advancements in delivery devices.
-
Patent expirations and regulatory pathways have facilitated an influx of generics, prompting significant price competition and shrinking profit margins for innovator brands.
-
Emerging markets represent substantial growth opportunities, supported by increased healthcare access and local manufacturing.
-
Innovation focusing on digital inhalers and combination therapies offers avenues for differentiation and revenue expansion amid commoditization pressures.
-
Market participants must navigate regulatory complexities and optimize supply chains to sustain financial performance.
Conclusion
Albuterol sulfate’s financial trajectory reflects a mature but dynamic market rooted in its central role in respiratory therapy. While price erosion due to generics challenges traditional revenue models, technological progress and expanding global access sustain growth prospects. Stakeholders investing in innovation, digital integration, and emerging markets are best positioned for value creation in the evolving landscape.
FAQs
1. How have patent expiries affected the albuterol sulfate market?
Patent expiries, notably in 2015 for GSK’s Ventolin, led to an influx of generic formulations, significantly reducing product prices and profit margins. This competitive landscape prompted companies to innovate or expand into new indications to sustain revenues.
2. What technological advancements are shaping albuterol inhaler development?
Innovations include breath-actuated inhalers, combination therapies, and digital connected inhalers that track adherence, improve delivery efficiency, and facilitate data sharing with healthcare providers.
3. What are the growth prospects for albuterol sulfate in emerging markets?
Growth prospects are strong due to increasing respiratory disease prevalence, rising healthcare investments, and local manufacturing. Lower-cost generics further facilitate market entry and volume increases.
4. How might regulatory policies influence future market dynamics?
Policies promoting biosimilar and generic adoption could continue to pressure prices, while regulatory incentives for innovative delivery systems may encourage R&D investments. A nuanced regulatory environment will shape product development and pricing strategies.
5. What challenges do companies face in maintaining profitability amid market saturation?
Major challenges include price erosion from generics, intense competition, patent litigation, and supply chain disruptions. Diversification into new therapies, digital health, and emerging markets remains critical for profitability.
Sources:
[1] Research and Markets. "Global Inhalation Therapies Market Report," 2022.
[2] Centers for Disease Control and Prevention (CDC). "Asthma Data," 2022.