Latanoprost - Generic Drug Details
✉ Email this page to a colleague
What are the generic sources for latanoprost and what is the scope of patent protection?
Latanoprost
is the generic ingredient in six branded drugs marketed by Sun Pharm, Thea Pharma, Amring Pharms, Apotex Inc, Bausch And Lomb, Carnegie, Epic Pharma Llc, Eugia Pharma, Fdc Ltd, Sandoz, Somerset, Upjohn, and Alcon Labs Inc, and is included in fourteen NDAs. There are twenty-three patents protecting this compound. Additional information is available in the individual branded drug profile pages.Latanoprost has fifty-three patent family members in thirty-one countries.
There are twenty drug master file entries for latanoprost. Twelve suppliers are listed for this compound. There is one tentative approval for this compound.
Summary for latanoprost
| International Patents: | 53 |
| US Patents: | 23 |
| Tradenames: | 6 |
| Applicants: | 13 |
| NDAs: | 14 |
| Drug Master File Entries: | 20 |
| Finished Product Suppliers / Packagers: | 12 |
| Raw Ingredient (Bulk) Api Vendors: | 77 |
| Clinical Trials: | 190 |
| Patent Applications: | 7,181 |
| Formulation / Manufacturing: | see details |
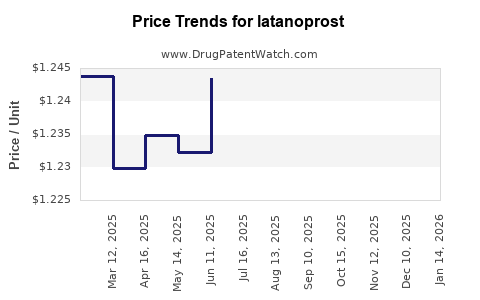
| Drug Prices: | Drug price trends for latanoprost |
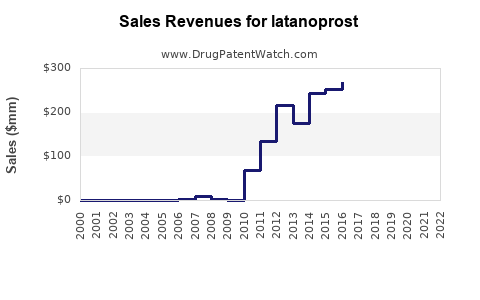
| Drug Sales Revenues: | Drug sales revenues for latanoprost |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for latanoprost |
| What excipients (inactive ingredients) are in latanoprost? | latanoprost excipients list |
| DailyMed Link: | latanoprost at DailyMed |
Recent Clinical Trials for latanoprost
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| DataPharm Australia, CMAX Clinical Research, Agilex Australia | Phase 1 |
| Aneira Pharma, Inc. | Phase 1 |
| Uzoma Chinyei Joan | Phase 4 |
Generic filers with tentative approvals for LATANOPROST
| Applicant | Application No. | Strength | Dosage Form |
| ⤷ Try a Trial | ⤷ Try a Trial | 0.005% | SOLUTION; OPHTHALMIC |
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
Pharmacology for latanoprost
| Drug Class | Prostaglandin Analog |
US Patents and Regulatory Information for latanoprost
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcon Labs Inc | ROCKLATAN | latanoprost; netarsudil dimesylate | SOLUTION/DROPS;OPHTHALMIC | 208259-001 | Mar 12, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Sun Pharm | XELPROS | latanoprost | EMULSION;OPHTHALMIC | 206185-001 | Sep 12, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Somerset | LATANOPROST | latanoprost | SOLUTION/DROPS;OPHTHALMIC | 201786-001 | Mar 22, 2011 | AT | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Alcon Labs Inc | ROCKLATAN | latanoprost; netarsudil dimesylate | SOLUTION/DROPS;OPHTHALMIC | 208259-001 | Mar 12, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for latanoprost
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Upjohn | XALATAN | latanoprost | SOLUTION/DROPS;OPHTHALMIC | 020597-001 | Jun 5, 1996 | ⤷ Try a Trial | ⤷ Try a Trial |
| Upjohn | XALATAN | latanoprost | SOLUTION/DROPS;OPHTHALMIC | 020597-001 | Jun 5, 1996 | ⤷ Try a Trial | ⤷ Try a Trial |
| Upjohn | XALATAN | latanoprost | SOLUTION/DROPS;OPHTHALMIC | 020597-001 | Jun 5, 1996 | ⤷ Try a Trial | ⤷ Try a Trial |
| Upjohn | XALATAN | latanoprost | SOLUTION/DROPS;OPHTHALMIC | 020597-001 | Jun 5, 1996 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for latanoprost
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Africa | 201002615 | OPHTHALMIC COMPOSITION COMPRISING A PROSTAGLANDIN | ⤷ Try a Trial |
| Colombia | 6270384 | COMPOSICIONES OFTALMICAS QUE COMPRENDEN DERIVADOS DE PROSTAGLANDINA | ⤷ Try a Trial |
| Japan | 5877885 | ⤷ Try a Trial | |
| Eurasian Patent Organization | 201070483 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for latanoprost
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0364417 | SPC/GB97/014 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: LATANOPROST (I.E. 13,14-DIHYDRO-17-PHENYL-18,19,20-TRINOR-PGF-ALPHA-ISOPROPYLESTER); NAT. REGISTRATION NO/DATE: 00032/0220 19961216; FIRST REGISTRATION: SE 12716 19960718; SPC EXTENSION AUTHORISATION: PL00057/1057-008 20101216 |
| 3461484 | 21C1024 | France | ⤷ Try a Trial | PRODUCT NAME: ASSOCIATION DE NETARSUDIL OU L'UN DE SES SELS ET DE LATANOPROST; REGISTRATION NO/DATE: EU/1/20/1502 20210108 |
| 0364417 | 61/1997 | Austria | ⤷ Try a Trial | PRODUCT NAME: LATANOPROST UND SEINE THERAPEUTISCH AKTIVEN UND PHYSIOLOGISCH ANNEHMBAREN DERIVATE; NAT. REGISTRATION NO/DATE: 1-22019 19970627; FIRST REGISTRATION: SE 12716 19960718 |
| 0364417 | C970039 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: [1R-[1 ALPHA (Z),2 BETA(R*),3 ALPHA,5ALPHA]]-7-[3,5-DIHYDROXY-2-(3-HYDROXY-5- FENYLPENTYL)CYCLOPENTYL]-5-HEPTEENZUUR, DESGEWENST IN DE VORM VAN EEN ZOUT OF EEN ESTER, IN HET BIJZONDER LATANOPROSTUM; NAT. REGISTRATION NO/DATE: RVG 21304 19970610; FIRST REGISTRATION: SE 12716 19960718 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.