Fentanyl - Generic Drug Details
✉ Email this page to a colleague
What are the generic sources for fentanyl and what is the scope of freedom to operate?
Fentanyl
is the generic ingredient in twenty-five branded drugs marketed by Janssen Pharms, Actavis Labs Ut Inc, Difgen Pharms, Kindeva, Lavipharm Labs, Mayne Pharma, Mylan Technologies, Noven, Specgx Llc, Zydus Pharms, Btcp Pharma, Adalvo, Abbott, Fresenius Kabi Usa, Hikma, Hospira, Watson Labs, Dr Reddys, Rising, Exela Pharma, Dr Reddys Labs Sa, Cephalon, Sentynl Theraps Inc, Actavis Labs Fl Inc, Par Pharm, and The Medicines Co, and is included in thirty-four NDAs. There are twenty-eight patents protecting this compound and one Paragraph IV challenge. Additional information is available in the individual branded drug profile pages.Fentanyl has thirty-two patent family members in seventeen countries.
There are thirty-one drug master file entries for fentanyl. Eight suppliers are listed for this compound. There are two tentative approvals for this compound.
Summary for fentanyl
| International Patents: | 32 |
| US Patents: | 28 |
| Tradenames: | 25 |
| Applicants: | 26 |
| NDAs: | 34 |
| Drug Master File Entries: | 31 |
| Finished Product Suppliers / Packagers: | 8 |
| Raw Ingredient (Bulk) Api Vendors: | 38 |
| Clinical Trials: | 1,654 |
| Patent Applications: | 6,998 |
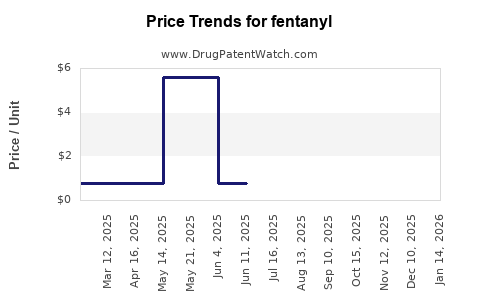
| Drug Prices: | Drug price trends for fentanyl |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for fentanyl |
| What excipients (inactive ingredients) are in fentanyl? | fentanyl excipients list |
| DailyMed Link: | fentanyl at DailyMed |
Recent Clinical Trials for fentanyl
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Alabama at Birmingham | Phase 1 |
| Merck Sharp & Dohme LLC | N/A |
| University Hospital "Sestre Milosrdnice" | N/A |
Generic filers with tentative approvals for FENTANYL
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
Pharmacology for fentanyl
| Drug Class | Opioid Agonist |
| Mechanism of Action | Full Opioid Agonists |
Medical Subject Heading (MeSH) Categories for fentanyl
Anatomical Therapeutic Chemical (ATC) Classes for fentanyl
Paragraph IV (Patent) Challenges for FENTANYL
US Patents and Regulatory Information for fentanyl
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sentynl Theraps Inc | ABSTRAL | fentanyl citrate | TABLET;SUBLINGUAL | 022510-006 | Jan 7, 2011 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Mylan Technologies | FENTANYL-100 | fentanyl | FILM, EXTENDED RELEASE;TRANSDERMAL | 076258-004 | Jan 28, 2005 | AB | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Hikma | FENTANYL CITRATE | fentanyl citrate | INJECTABLE;INJECTION | 019101-002 | Jan 20, 2023 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Cephalon | FENTORA | fentanyl citrate | TABLET;BUCCAL, SUBLINGUAL | 021947-001 | Sep 25, 2006 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Btcp Pharma | SUBSYS | fentanyl | SPRAY;SUBLINGUAL | 202788-003 | Jan 4, 2012 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for fentanyl
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Janssen Pharms | DURAGESIC-50 | fentanyl | FILM, EXTENDED RELEASE;TRANSDERMAL | 019813-003 | Aug 7, 1990 | ⤷ Sign Up | ⤷ Sign Up |
| Janssen Pharms | DURAGESIC-75 | fentanyl | FILM, EXTENDED RELEASE;TRANSDERMAL | 019813-002 | Aug 7, 1990 | ⤷ Sign Up | ⤷ Sign Up |
| Janssen Pharms | DURAGESIC-100 | fentanyl | FILM, EXTENDED RELEASE;TRANSDERMAL | 019813-001 | Aug 7, 1990 | ⤷ Sign Up | ⤷ Sign Up |
| Janssen Pharms | DURAGESIC-50 | fentanyl | FILM, EXTENDED RELEASE;TRANSDERMAL | 019813-003 | Aug 7, 1990 | ⤷ Sign Up | ⤷ Sign Up |
| Janssen Pharms | DURAGESIC-50 | fentanyl | FILM, EXTENDED RELEASE;TRANSDERMAL | 019813-003 | Aug 7, 1990 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for fentanyl
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Kyowa Kirin Holdings B.V. | PecFent | fentanyl | EMEA/H/C/001164 PecFent is indicated for the management of breakthrough pain in adults who are already receiving maintenance opioid therapy for chronic cancer pain. Breakthrough pain is a transitory exacerbation of pain that occurs on a background of otherwise controlled persistent pain.Patients receiving maintenance opioid therapy are those who are taking at least 60 mg of oral morphine daily, at least 25 micrograms of transdermal fentanyl per hour, at least 30 mg of oxycodone daily, at least 8 mg of oral hydromorphone daily or an equi-analgesic dose of another opioid for a week or longer. |
Authorised | no | no | no | 2010-08-31 | |
| Takeda Pharma A/S | Instanyl | fentanyl | EMEA/H/C/000959 Instanyl is indicated for the management of breakthrough pain in adults already receiving maintenance opioid therapy for chronic cancer pain. Breakthrough pain is a transitory exacerbation of pain that occurs on a background of otherwise controlled persistent pain. Patients receiving maintenance opioid therapy are those who are taking at least 60 mg of oral morphine daily, at least 25 micrograms of transdermal fentanyl per hour, at least 30 mg oxycodone daily, at least 8 mg of oral hydromorphone daily or an equianalgesic dose of another opioid for a week or longer. |
Authorised | no | no | no | 2009-07-20 | |
| Teva B.V. | Effentora | fentanyl | EMEA/H/C/000833 Effentora is indicated for the treatment of breakthrough pain (BTP) in adults with cancer who are already receiving maintenance opioid therapy for chronic cancer pain., , BTP is a transitory exacerbation of pain that occurs on a background of otherwise controlled persistent pain., , Patients receiving maintenance opioid therapy are those who are taking at least 60 mg of oral morphine daily, at least 25 micrograms of transdermal fentanyl per hour, at least 30 mg of oxycodone daily, at least 8 mg of oral hydromorphone daily or an equianalgesic dose of another opioid for a week or longer. , |
Authorised | no | no | no | 2008-04-04 | |
| Incline Therapeutics Europe Ltd | Ionsys | fentanyl | EMEA/H/C/002715 Ionsys is indicated for the management of acute moderate to severe post-operative pain in adult patients. |
Withdrawn | no | no | no | 2015-11-18 | |
| Eli Lilly and Company Limited | Recuvyra | fentanyl | EMEA/V/C/002239 For the control of pain associated with orthopaedic and soft tissue surgery in dogs. |
Withdrawn | no | no | no | 2011-10-06 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for fentanyl
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 1976521 | ⤷ Sign Up | |
| Japan | 2009528272 | ⤷ Sign Up | |
| Japan | 2013056928 | ⤷ Sign Up | |
| Russian Federation | 2432950 | SUBLINGUAL FENTANYL-BASED SPRAY | ⤷ Sign Up |
| Canada | 2637672 | SUBLINGUAL FENTANYL SPRAY | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for fentanyl
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0383579 | C960030 | Netherlands | ⤷ Sign Up | PRODUCT NAME: REMIFENTANYLUM, DESGEWENST IN DE VORM VAN EEN ZUURADDITIE-ZOUT, IN HET BIJZONDER HET HYDROCHLORIDE; NAT. REGISTRATION NO/DATE: RVG 20601 - RVG 20603 19961015; 36335.00.00, 36335.01.00, 36335.02.00 19960517 |
| 0901368 | C300523 | Netherlands | ⤷ Sign Up | PRODUCT NAME: FENTANYL; REGISTRATION NO/DATE: EU/2/11/127/001 20111006 |
| 1769785 | C300522 | Netherlands | ⤷ Sign Up | PRODUCT NAME: FENTANYL EN DOSERINGSAPPLICATOR; REG. NO/DATE: EU/2/11/127/001 20111006 |
| 0975367 | 122011000009 | Germany | ⤷ Sign Up | PRODUCT NAME: FENTANYL IN ALLEN DEM SCHUTZ DES GRUNDPATENTS UNTERLIEGENDEN FORMEN; REGISTRATION NO/DATE: EU/1/10/644/001-004 20100831 |
| 1635783 | CA 2014 00016 | Denmark | ⤷ Sign Up | PRODUCT NAME: FENTANYL I EN HVILKEN SOM HELST AF DE FORMER, DER ER BESKYTTET AF GRUNDPATENTET; REG. NO/DATE: EU/1/10/644/001-006 20100831 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.