Everolimus - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for everolimus and what is the scope of patent protection?
Everolimus
is the generic ingredient in four branded drugs marketed by Novartis Pharm, Mylan, Novartis, Alkem Labs Ltd, Biocon Pharma, Breckenridge, Endo Operations, Hikma, and Teva Pharms Usa, and is included in fourteen NDAs. There are two patents protecting this compound and four Paragraph IV challenges. Additional information is available in the individual branded drug profile pages.Everolimus has two hundred and twenty-nine patent family members in thirty-one countries.
There are twelve drug master file entries for everolimus. Ten suppliers are listed for this compound.
Summary for everolimus
| International Patents: | 229 |
| US Patents: | 2 |
| Tradenames: | 4 |
| Applicants: | 9 |
| NDAs: | 14 |
| Drug Master File Entries: | 12 |
| Finished Product Suppliers / Packagers: | 10 |
| Raw Ingredient (Bulk) Api Vendors: | 38 |
| Clinical Trials: | 779 |
| Patent Applications: | 6,570 |
| Formulation / Manufacturing: | see details |
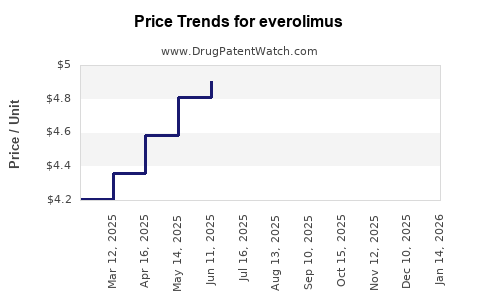
| Drug Prices: | Drug price trends for everolimus |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for everolimus |
| What excipients (inactive ingredients) are in everolimus? | everolimus excipients list |
| DailyMed Link: | everolimus at DailyMed |
Recent Clinical Trials for everolimus
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Stanford University | Phase 1/Phase 2 |
| Central Hospital, Nancy, France | Phase 2 |
| Pfizer | Phase 3 |
Pharmacology for everolimus
| Drug Class | Kinase Inhibitor mTOR Inhibitor Immunosuppressant |
| Mechanism of Action | Cytochrome P450 2D6 Inhibitors Cytochrome P450 3A4 Inhibitors Protein Kinase Inhibitors mTOR Inhibitors |
| Physiological Effect | Decreased Immunologic Activity |
Medical Subject Heading (MeSH) Categories for everolimus
Anatomical Therapeutic Chemical (ATC) Classes for everolimus
Paragraph IV (Patent) Challenges for EVEROLIMUS
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| AFINITOR DISPERZ | Tablets for Oral Suspension | everolimus | 2 mg, 3 mg and 5 mg | 203985 | 1 | 2016-12-30 |
| AFINITOR | Tablets | everolimus | 2.5 mg, 5 mg, and 7.5 mg | 022334 | 1 | 2014-12-10 |
| AFINITOR | Tablets | everolimus | 10 mg | 022334 | 1 | 2014-06-18 |
| ZORTRESS | Tablets | everolimus | 0.25 mg, 0.5 mg, and 0.75 mg | 021560 | 3 | 2013-09-30 |
US Patents and Regulatory Information for everolimus
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novartis | AFINITOR | everolimus | TABLET;ORAL | 022334-001 | Mar 30, 2009 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Novartis | ZORTRESS | everolimus | TABLET;ORAL | 021560-001 | Apr 20, 2010 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Novartis | AFINITOR | everolimus | TABLET;ORAL | 022334-004 | Mar 30, 2012 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Endo Operations | EVEROLIMUS | everolimus | TABLET;ORAL | 207934-001 | Dec 9, 2019 | AB | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for everolimus
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Novartis | ZORTRESS | everolimus | TABLET;ORAL | 021560-001 | Apr 20, 2010 | ⤷ Sign Up | ⤷ Sign Up |
| Novartis | ZORTRESS | everolimus | TABLET;ORAL | 021560-003 | Apr 20, 2010 | ⤷ Sign Up | ⤷ Sign Up |
| Novartis | AFINITOR | everolimus | TABLET;ORAL | 022334-001 | Mar 30, 2009 | ⤷ Sign Up | ⤷ Sign Up |
| Novartis Pharm | AFINITOR DISPERZ | everolimus | TABLET, FOR SUSPENSION;ORAL | 203985-002 | Aug 29, 2012 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for everolimus
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Novartis Europharm Limited | Afinitor | everolimus | EMEA/H/C/001038 Hormone-receptor-positive advanced breast cancerAfinitor is indicated for the treatment of hormone-receptor-positive, HER2/neu-negative advanced breast cancer, in combination with exemestane, in post-menopausal women without symptomatic visceral disease after recurrence or progression following a non-steroidal aromatase inhibitor.Neuroendocrine tumours of pancreatic originAfinitor is indicated for the treatment of unresectable or metastatic, well or moderately differentiated neuroendocrine tumours of pancreatic origin in adults with progressive disease.Neuroendocrine tumours of gastrointestinal or lung originAfinitor is indicated for the treatment of unresectable or metastatic, well-differentiated (Grade 1 or Grade 2) non-functional neuroendocrine tumours of gastrointestinal or lung origin in adults with progressive disease.Renal-cell carcinomaAfinitor is indicated for the treatment of patients with advanced renal-cell carcinoma, whose disease has progressed on or after treatment with VEGF-targeted therapy. |
Authorised | no | no | no | 2009-08-02 | |
| Novartis Europharm Limited | Votubia | everolimus | EMEA/H/C/002311 Renal angiomyolipoma associated with tuberous sclerosis complex (TSC)Votubia is indicated for the treatment of adult patients with renal angiomyolipoma associated with tuberous sclerosis complex (TSC) who are at risk of complications (based on factors such as tumour size or presence of aneurysm, or presence of multiple or bilateral tumours) but who do not require immediate surgery.The evidence is based on analysis of change in sum of angiomyolipoma volume.Subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC)Votubia is indicated for the treatment of patients with subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC) who require therapeutic intervention but are not amenable to surgery.The evidence is based on analysis of change in SEGA volume. Further clinical benefit, such as improvement in disease‑related symptoms, has not been demonstrated. |
Authorised | no | no | no | 2011-09-02 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for everolimus
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Slovakia | 10382003 | Použitie rapamycinu na prípravu farmaceutickej kompozície na použitie na liečenie pevných nádorov (Cancer treatment) | ⤷ Sign Up |
| Spain | 2640787 | ⤷ Sign Up | |
| Norway | 20170956 | Neuroendokrin tumorbehandling | ⤷ Sign Up |
| Norway | 20033651 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for everolimus
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0663916 | 2010/001 | Ireland | ⤷ Sign Up | PRODUCT NAME: EVEROLIMUS; NAT REGISTRATION NO/DATE: EU/1/09/538/001-006 20090803; FIRST REGISTRATION NO/DATE: 18960 018691 18962 18693 18694 18695. 20030718 |
| 2269603 | CA 2015 00058 | Denmark | ⤷ Sign Up | PRODUCT NAME: EVEROLIMUS ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF; REG. NO/DATE: EU/1/09/538/001-008 (K(2012)5347) 20120725 |
| 2269603 | 132015000073030 | Italy | ⤷ Sign Up | PRODUCT NAME: EVEROLIMUS O UN SUO SALE FARMACEUTICAMENTE ACCCETTABILE(AFINITOR); AUTHORISATION NUMBER(S) AND DATE(S): C(2012)5347, 20120725 |
| 2269604 | PA2016035 | Lithuania | ⤷ Sign Up | PRODUCT NAME: EVEROLIMUZAS; REGISTRATION NO/DATE: EU/1/09/538/001-006 20090803 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.