EVEROLIMUS Drug Patent Profile
✉ Email this page to a colleague
When do Everolimus patents expire, and what generic alternatives are available?
Everolimus is a drug marketed by Amneal, Mylan, Natco, Alkem Labs Ltd, Biocon Pharma, Breckenridge, Hikma, Novugen, Ph Health, and Teva Pharms Usa. and is included in fifteen NDAs.
The generic ingredient in EVEROLIMUS is everolimus. There are twelve drug master file entries for this compound. Twelve suppliers are listed for this compound. Additional details are available on the everolimus profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Everolimus
A generic version of EVEROLIMUS was approved as everolimus by HIKMA on April 12th, 2018.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for EVEROLIMUS?
- What are the global sales for EVEROLIMUS?
- What is Average Wholesale Price for EVEROLIMUS?
Summary for EVEROLIMUS
| US Patents: | 0 |
| Applicants: | 10 |
| NDAs: | 15 |
| Finished Product Suppliers / Packagers: | 11 |
| Raw Ingredient (Bulk) Api Vendors: | 41 |
| Clinical Trials: | 818 |
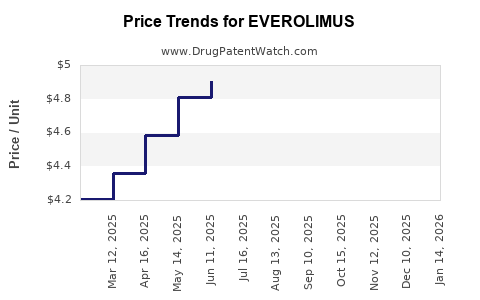
| Drug Prices: | Drug price information for EVEROLIMUS |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for EVEROLIMUS |
| DailyMed Link: | EVEROLIMUS at DailyMed |
Recent Clinical Trials for EVEROLIMUS
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Boston Children's Hospital | PHASE2 |
| Cancer Institute and Hospital, Chinese Academy of Medical Sciences | PHASE3 |
| University of Texas Southwestern Medical Center | PHASE1 |
Pharmacology for EVEROLIMUS
| Drug Class | Kinase Inhibitor mTOR Inhibitor Immunosuppressant |
| Mechanism of Action | Cytochrome P450 2D6 Inhibitors Cytochrome P450 3A4 Inhibitors Protein Kinase Inhibitors mTOR Inhibitors |
| Physiological Effect | Decreased Immunologic Activity |
Medical Subject Heading (MeSH) Categories for EVEROLIMUS
Anatomical Therapeutic Chemical (ATC) Classes for EVEROLIMUS
Paragraph IV (Patent) Challenges for EVEROLIMUS
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| AFINITOR DISPERZ | Tablets for Oral Suspension | everolimus | 2 mg, 3 mg and 5 mg | 203985 | 1 | 2016-12-30 |
| AFINITOR | Tablets | everolimus | 2.5 mg, 5 mg, and 7.5 mg | 022334 | 1 | 2014-12-10 |
| AFINITOR | Tablets | everolimus | 10 mg | 022334 | 1 | 2014-06-18 |
| ZORTRESS | Tablets | everolimus | 0.25 mg, 0.5 mg, and 0.75 mg | 021560 | 3 | 2013-09-30 |
US Patents and Regulatory Information for EVEROLIMUS
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mylan | EVEROLIMUS | everolimus | TABLET;ORAL | 212936-004 | Jun 8, 2020 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Hikma | EVEROLIMUS | everolimus | TABLET;ORAL | 206133-003 | Apr 12, 2018 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Ph Health | EVEROLIMUS | everolimus | TABLET;ORAL | 205775-004 | Oct 18, 2021 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Novugen | EVEROLIMUS | everolimus | TABLET;ORAL | 219955-004 | Oct 30, 2025 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Ph Health | EVEROLIMUS | everolimus | TABLET;ORAL | 207934-001 | Dec 9, 2019 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for EVEROLIMUS
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Novartis Europharm Limited | Afinitor | everolimus | EMEA/H/C/001038Hormone-receptor-positive advanced breast cancerAfinitor is indicated for the treatment of hormone-receptor-positive, HER2/neu-negative advanced breast cancer, in combination with exemestane, in post-menopausal women without symptomatic visceral disease after recurrence or progression following a non-steroidal aromatase inhibitor.Neuroendocrine tumours of pancreatic originAfinitor is indicated for the treatment of unresectable or metastatic, well or moderately differentiated neuroendocrine tumours of pancreatic origin in adults with progressive disease.Neuroendocrine tumours of gastrointestinal or lung originAfinitor is indicated for the treatment of unresectable or metastatic, well-differentiated (Grade 1 or Grade 2) non-functional neuroendocrine tumours of gastrointestinal or lung origin in adults with progressive disease.Renal-cell carcinomaAfinitor is indicated for the treatment of patients with advanced renal-cell carcinoma, whose disease has progressed on or after treatment with VEGF-targeted therapy. | Authorised | no | no | no | 2009-08-02 | |
| Novartis Europharm Limited | Votubia | everolimus | EMEA/H/C/002311Renal angiomyolipoma associated with tuberous sclerosis complex (TSC)Votubia is indicated for the treatment of adult patients with renal angiomyolipoma associated with tuberous sclerosis complex (TSC) who are at risk of complications (based on factors such as tumour size or presence of aneurysm, or presence of multiple or bilateral tumours) but who do not require immediate surgery.The evidence is based on analysis of change in sum of angiomyolipoma volume.Subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC)Votubia is indicated for the treatment of patients with subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC) who require therapeutic intervention but are not amenable to surgery.The evidence is based on analysis of change in SEGA volume. Further clinical benefit, such as improvement in disease‑related symptoms, has not been demonstrated. | Authorised | no | no | no | 2011-09-02 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
Market Dynamics and Financial Trajectory for Everolimus
More… ↓
