LATANOPROST - Generic Drug Details
✉ Email this page to a colleague
What are the generic sources for latanoprost and what is the scope of freedom to operate?
Latanoprost
is the generic ingredient in seven branded drugs marketed by Sun Pharm, Thea Pharma, Amring Pharms, Apotex Inc, Bausch And Lomb, Epic Pharma Llc, Eugia Pharma, Fdc Ltd, Gland, Micro Labs, Pharmobedient, Sandoz, Somerset, Upjohn, and Alcon Labs Inc, and is included in seventeen NDAs. There are twenty-four patents protecting this compound. Additional information is available in the individual branded drug profile pages.Latanoprost has fifty-three patent family members in thirty-one countries.
There are twenty drug master file entries for latanoprost. Fifteen suppliers are listed for this compound. There is one tentative approval for this compound.
Summary for LATANOPROST
| International Patents: | 53 |
| US Patents: | 24 |
| Tradenames: | 7 |
| Applicants: | 15 |
| NDAs: | 17 |
| Drug Master File Entries: | 20 |
| Finished Product Suppliers / Packagers: | 15 |
| Raw Ingredient (Bulk) Api Vendors: | 77 |
| Clinical Trials: | 204 |
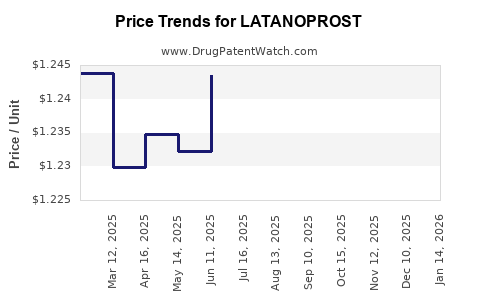
| Drug Prices: | Drug price trends for LATANOPROST |
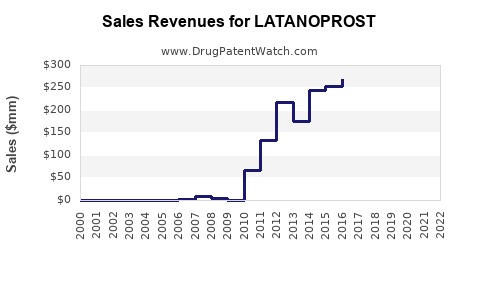
| Drug Sales Revenues: | Drug sales revenues for LATANOPROST |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for LATANOPROST |
| What excipients (inactive ingredients) are in LATANOPROST? | LATANOPROST excipients list |
| DailyMed Link: | LATANOPROST at DailyMed |
Recent Clinical Trials for LATANOPROST
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Insight Eyecare Specialties, Inc. dba Vision Source Eyecare, | PHASE4 |
| Universitas Padjadjaran | PHASE2 |
| PolyActiva Pty Ltd | PHASE2 |
Generic filers with tentative approvals for LATANOPROST
| Applicant | Application No. | Strength | Dosage Form |
| ⤷ Get Started Free | ⤷ Get Started Free | 0.005% | SOLUTION; OPHTHALMIC |
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
Anatomical Therapeutic Chemical (ATC) Classes for LATANOPROST
US Patents and Regulatory Information for LATANOPROST
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gland | LATANOPROSTENE BUNOD | latanoprostene bunod | SOLUTION/DROPS;OPHTHALMIC | 217387-001 | Apr 29, 2025 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Alcon Labs Inc | ROCKLATAN | latanoprost; netarsudil dimesylate | SOLUTION/DROPS;OPHTHALMIC | 208259-001 | Mar 12, 2019 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Alcon Labs Inc | ROCKLATAN | latanoprost; netarsudil dimesylate | SOLUTION/DROPS;OPHTHALMIC | 208259-001 | Mar 12, 2019 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Bausch And Lomb | VYZULTA | latanoprostene bunod | SOLUTION/DROPS;OPHTHALMIC | 207795-001 | Nov 2, 2017 | AB | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | Y | ⤷ Get Started Free | ||
| Alcon Labs Inc | ROCKLATAN | latanoprost; netarsudil dimesylate | SOLUTION/DROPS;OPHTHALMIC | 208259-001 | Mar 12, 2019 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for LATANOPROST
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Upjohn | XALATAN | latanoprost | SOLUTION/DROPS;OPHTHALMIC | 020597-001 | Jun 5, 1996 | ⤷ Get Started Free | ⤷ Get Started Free |
| Upjohn | XALATAN | latanoprost | SOLUTION/DROPS;OPHTHALMIC | 020597-001 | Jun 5, 1996 | ⤷ Get Started Free | ⤷ Get Started Free |
| Upjohn | XALATAN | latanoprost | SOLUTION/DROPS;OPHTHALMIC | 020597-001 | Jun 5, 1996 | ⤷ Get Started Free | ⤷ Get Started Free |
| Upjohn | XALATAN | latanoprost | SOLUTION/DROPS;OPHTHALMIC | 020597-001 | Jun 5, 1996 | ⤷ Get Started Free | ⤷ Get Started Free |
| Upjohn | XALATAN | latanoprost | SOLUTION/DROPS;OPHTHALMIC | 020597-001 | Jun 5, 1996 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for LATANOPROST
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Ecuador | SP10010167 | COMPOSICIONES OFTÁLMICAS NOVEDOSAS | ⤷ Get Started Free |
| Japan | 2011508724 | ⤷ Get Started Free | |
| Tunisia | 2012000588 | SYSTEME DE DELIVRANCE POLYMERIQUE D'UNE SOLUTION NON VISQUEUSE A BASE DE PROSTAGLANDINE SANS CONSERVATEUR | ⤷ Get Started Free |
| Poland | 2588078 | ⤷ Get Started Free | |
| Eurasian Patent Organization | 201070483 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for LATANOPROST
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3461484 | 132021000000068 | Italy | ⤷ Get Started Free | PRODUCT NAME: COMBINAZIONE DI LATANOPROST E NETARSUDIL(ROCLANDA); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/20/1502, 20210108 |
| 3461484 | C202130024 | Spain | ⤷ Get Started Free | PRODUCT NAME: NETARSUDIL O UN ENANTIOMERO, DIASTEREIOISOMERO, SAL O SALVADO DEL MISMO EN COMBINACION CON LATANOPROST O UNA SAL DEL MISMO; NATIONAL AUTHORISATION NUMBER: EU/1/20/1502; DATE OF AUTHORISATION: 20210107; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/20/1502; DATE OF FIRST AUTHORISATION IN EEA: 20210107 |
| 0364417 | 61/1997 | Austria | ⤷ Get Started Free | PRODUCT NAME: LATANOPROST UND SEINE THERAPEUTISCH AKTIVEN UND PHYSIOLOGISCH ANNEHMBAREN DERIVATE; NAT. REGISTRATION NO/DATE: 1-22019 19970627; FIRST REGISTRATION: SE 12716 19960718 |
| 3461484 | 301101 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: LATANOPROST OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN EN NETARSUDILMESYLAAT; REGISTRATION NO/DATE: EU/1/20/1502 20210108 |
| 0364417 | C970039 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: [1R-[1 ALPHA (Z),2 BETA(R*),3 ALPHA,5ALPHA]]-7-[3,5-DIHYDROXY-2-(3-HYDROXY-5- FENYLPENTYL)CYCLOPENTYL]-5-HEPTEENZUUR, DESGEWENST IN DE VORM VAN EEN ZOUT OF EEN ESTER, IN HET BIJZONDER LATANOPROSTUM; NAT. REGISTRATION NO/DATE: RVG 21304 19970610; FIRST REGISTRATION: SE 12716 19960718 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for Latanoprost
More… ↓