DAPAGLIFLOZIN - Generic Drug Details
✉ Email this page to a colleague
What are the generic sources for dapagliflozin and what is the scope of patent protection?
Dapagliflozin
is the generic ingredient in four branded drugs marketed by Astrazeneca Ab and is included in four NDAs. There are twenty-one patents protecting this compound. Additional information is available in the individual branded drug profile pages.Dapagliflozin has four hundred and fifty patent family members in fifty-two countries.
There are twenty-six drug master file entries for dapagliflozin. Five suppliers are listed for this compound. There are sixteen tentative approvals for this compound.
Summary for DAPAGLIFLOZIN
| International Patents: | 450 |
| US Patents: | 21 |
| Tradenames: | 4 |
| Applicants: | 1 |
| NDAs: | 4 |
| Drug Master File Entries: | 26 |
| Finished Product Suppliers / Packagers: | 5 |
| Raw Ingredient (Bulk) Api Vendors: | 84 |
| Clinical Trials: | 542 |
| Patent Applications: | 6,063 |
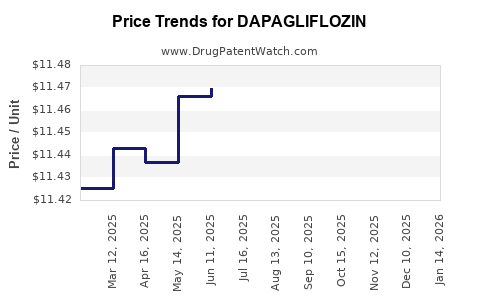
| Drug Prices: | Drug price trends for DAPAGLIFLOZIN |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for DAPAGLIFLOZIN |
| What excipients (inactive ingredients) are in DAPAGLIFLOZIN? | DAPAGLIFLOZIN excipients list |
| DailyMed Link: | DAPAGLIFLOZIN at DailyMed |
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for DAPAGLIFLOZIN
Generic Entry Date for DAPAGLIFLOZIN*:
Constraining patent/regulatory exclusivity:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for DAPAGLIFLOZIN
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Aberdeen | NA |
| Centre Hospitalier Universitaire de Nice | PHASE2 |
| NHS Grampian | NA |
Generic filers with tentative approvals for DAPAGLIFLOZIN
| Applicant | Application No. | Strength | Dosage Form |
| ⤷ Get Started Free | ⤷ Get Started Free | 2.5MG;1GM | TABLET, EXTENDED RELEASE;ORAL |
| ⤷ Get Started Free | ⤷ Get Started Free | 10MG;1GM | TABLET, EXTENDED RELEASE;ORAL |
| ⤷ Get Started Free | ⤷ Get Started Free | 10MG;500MG | TABLET, EXTENDED RELEASE;ORAL |
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
Pharmacology for DAPAGLIFLOZIN
| Drug Class | Sodium-Glucose Cotransporter 2 Inhibitor |
| Mechanism of Action | Sodium-Glucose Transporter 2 Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for DAPAGLIFLOZIN
Paragraph IV (Patent) Challenges for DAPAGLIFLOZIN
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| FARXIGA | Tablets | dapagliflozin | 5 mg and 10 mg | 202293 | 20 | 2018-01-08 |
US Patents and Regulatory Information for DAPAGLIFLOZIN
Expired US Patents for DAPAGLIFLOZIN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Astrazeneca Ab | FARXIGA | dapagliflozin | TABLET;ORAL | 202293-001 | Jan 8, 2014 | ⤷ Get Started Free | ⤷ Get Started Free |
| Astrazeneca Ab | FARXIGA | dapagliflozin | TABLET;ORAL | 202293-002 | Jan 8, 2014 | ⤷ Get Started Free | ⤷ Get Started Free |
| Astrazeneca Ab | FARXIGA | dapagliflozin | TABLET;ORAL | 202293-002 | Jan 8, 2014 | ⤷ Get Started Free | ⤷ Get Started Free |
| Astrazeneca Ab | FARXIGA | dapagliflozin | TABLET;ORAL | 202293-002 | Jan 8, 2014 | ⤷ Get Started Free | ⤷ Get Started Free |
| Astrazeneca Ab | FARXIGA | dapagliflozin | TABLET;ORAL | 202293-002 | Jan 8, 2014 | ⤷ Get Started Free | ⤷ Get Started Free |
| Astrazeneca Ab | FARXIGA | dapagliflozin | TABLET;ORAL | 202293-002 | Jan 8, 2014 | ⤷ Get Started Free | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for DAPAGLIFLOZIN
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| AstraZeneca AB | Forxiga | dapagliflozin | EMEA/H/C/002322Type 2 diabetes mellitusForxiga is indicated in adults and children aged 10 years and above for the treatment of insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exerciseas monotherapy when metformin is considered inappropriate due to intolerance.in addition to other medicinal products for the treatment of type 2 diabetes.For study results with respect to combination of therapies, effects on glycaemic control, cardiovascular and renal events, and the populations studied, see sections 4.4, 4.5 and 5.1.Heart failureForxiga is indicated in adults for the treatment of symptomatic chronic heart failure.Chronic kidney diseaseForxiga is indicated in adults for the treatment of chronic kidney disease. | Authorised | no | no | no | 2012-11-11 | |
| AstraZeneca AB | Edistride | dapagliflozin | EMEA/H/C/004161Type 2 diabetes mellitusEdistride is indicated in adults and children aged 10 years and above for the treatment of insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exerciseas monotherapy when metformin is considered inappropriate due to intolerance.in addition to other medicinal products for the treatment of type 2 diabetes.For study results with respect to combination of therapies, effects on glycaemic control, cardiovascular and renal events, and the populations studied, see sections 4.4, 4.5 and 5.1.Heart failureEdistride is indicated in adults for the treatment of symptomatic chronic heart failure.Chronic kidney diseaseEdistride is indicated in adults for the treatment of chronic kidney disease. | Authorised | no | no | no | 2015-11-09 | |
| Viatris Limited | Dapagliflozin Viatris | dapagliflozin | EMEA/H/C/006006Type 2 diabetes mellitusDapagliflozin Viatris is indicated in adults and children aged 10 years and above for the treatment of insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise- as monotherapy when metformin is considered inappropriate due to intolerance.- in addition to other medicinal products for the treatment of type 2 diabetes.For study results with respect to combination of therapies, effects on glycaemic control, cardiovascular and renal events, and the populations studied, see sections 4.4, 4.5 and 5.1.Heart failureDapagliflozin Viatris is indicated in adults for the treatment of symptomatic chronic heart failure with reduced ejection fraction.Chronic kidney diseaseDapagliflozin Viatris is indicated in adults for the treatment of chronic kidney disease. | Authorised | yes | no | no | 2023-03-24 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for DAPAGLIFLOZIN
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Lithuania | 3524261 | ⤷ Get Started Free | |
| Spain | 2950089 | ⤷ Get Started Free | |
| Denmark | 3524261 | ⤷ Get Started Free | |
| Japan | 4899021 | ⤷ Get Started Free | |
| Hong Kong | 1176004 | ⤷ Get Started Free | |
| European Patent Office | 1958623 | Microcapsules à libération prolongée à base de poly(Lactide-Co-Glycolide) comportant un polypeptide et un sucre (Poly(Lactide-Co-Glycolide)-based sustained release microcapsules comprising a polypeptide and a sugar) | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for DAPAGLIFLOZIN
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2139494 | C202030045 | Spain | ⤷ Get Started Free | PRODUCT NAME: SAXAGLIPTINA + DAPAGLIFOZINA; NATIONAL AUTHORISATION NUMBER: EU/1/16/1108; DATE OF AUTHORISATION: 20160715; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/16/1108; DATE OF FIRST AUTHORISATION IN EEA: 20160715 |
| 1506211 | 2013/013 | Ireland | ⤷ Get Started Free | PRODUCT NAME: DAPAGLIFLOZIN AND PHARACEUTICALLY ACCEPTABLE SALTS THREOF; REGISTRATION NO/DATE: EU/1/12/795/001-010 20121112 |
| 1506211 | PA2014026,C1506211 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: DAPAGLIFLOZINUM + METFORMINUM; REGISTRATION NO/DATE: EU/1/13/900 20140116 |
| 2139494 | 2020C/533 | Belgium | ⤷ Get Started Free | PRODUCT NAME: QTERN -SAXAGLIPTINE ET DAPAGLIFLOZINE; AUTHORISATION NUMBER AND DATE: EU/1/16/1108 20160719 |
| 2139494 | C20200028 00358 | Estonia | ⤷ Get Started Free | PRODUCT NAME: SAKSAGLIPTIIN JA DAPAGLIFLOSIIN;REG NO/DATE: EU/1/16/1108; 19.07.2016 |
| 1506211 | 132014902277722 | Italy | ⤷ Get Started Free | PRODUCT NAME: UNA COMBINAZIONE DI DAPAGLIFLOZIN O UN SUO SALE FARMACEUTICAMENTE ACCETTABILE E METFORMINA O UN SUO SALE FARMACEUTICAMENTE ACCETTABILE COME PROTETTI DAL BREVETTO DI BASE EP1506211(XIGDUO); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/13/900, 20140116 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for Dapagliflozin
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.