UCB INC Company Profile
✉ Email this page to a colleague
What is the competitive landscape for UCB INC, and what generic alternatives to UCB INC drugs are available?
UCB INC has forty-two approved drugs.
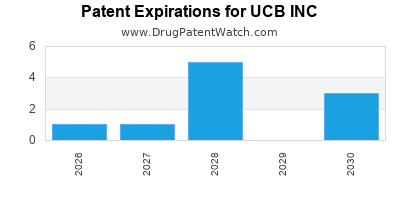
There are thirty-four US patents protecting UCB INC drugs.
There are four hundred and ninety patent family members on UCB INC drugs in fifty-one countries and forty-three supplementary protection certificates in fifteen countries.
Summary for UCB INC
| International Patents: | 490 |
| US Patents: | 34 |
| Tradenames: | 29 |
| Ingredients: | 24 |
| NDAs: | 42 |
| Drug Master File Entries: | 1 |
| Patent Litigation for UCB INC: | See patent lawsuits for UCB INC |
Drugs and US Patents for UCB INC
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ucb Inc | FINTEPLA | fenfluramine hydrochloride | SOLUTION;ORAL | 212102-001 | Jun 25, 2020 | RX | Yes | Yes | 10,603,290*PED | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Ucb Inc | BRIVIACT | brivaracetam | TABLET;ORAL | 205836-003 | May 12, 2016 | RX | Yes | No | 10,729,653 | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Ucb Inc | ZILBRYSQ | zilucoplan sodium | SOLUTION;SUBCUTANEOUS | 216834-003 | Oct 17, 2023 | RX | Yes | Yes | 10,106,579 | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Ucb Inc | VIMPAT | lacosamide | TABLET;ORAL | 022253-004 | Oct 28, 2008 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ||||
| Ucb Inc | ZILBRYSQ | zilucoplan sodium | SOLUTION;SUBCUTANEOUS | 216834-001 | Oct 17, 2023 | RX | Yes | Yes | 11,752,190 | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for UCB INC
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Ucb Inc | NEUPRO | rotigotine | FILM, EXTENDED RELEASE;TRANSDERMAL | 021829-001 | May 9, 2007 | 6,884,434 | ⤷ Sign Up |
| Ucb Inc | KEPPRA XR | levetiracetam | TABLET, EXTENDED RELEASE;ORAL | 022285-001 | Sep 12, 2008 | 4,943,639*PED | ⤷ Sign Up |
| Ucb Inc | UNIVASC | moexipril hydrochloride | TABLET;ORAL | 020312-002 | Apr 19, 1995 | 4,743,450 | ⤷ Sign Up |
| Ucb Inc | NIRAVAM | alprazolam | TABLET, ORALLY DISINTEGRATING;ORAL | 021726-001 | Jan 19, 2005 | 6,221,392 | ⤷ Sign Up |
| Ucb Inc | NEUPRO | rotigotine | FILM, EXTENDED RELEASE;TRANSDERMAL | 021829-002 | May 9, 2007 | 6,699,498 | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for UCB INC drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Oral Solution | 10 mg/mL | ➤ Subscribe | 2012-10-29 |
| ➤ Subscribe | Injection | 10 mg/mL, 20 mL | ➤ Subscribe | 2016-06-30 |
| ➤ Subscribe | Orally Disintegrating Tablets | 0.25 mg, 0.5 mg, 1 mg and 2 mg | ➤ Subscribe | 2005-12-27 |
| ➤ Subscribe | Tablets | 1000 mg | ➤ Subscribe | 2007-01-24 |
| ➤ Subscribe | Tablets | 50 mg, 100 mg, 150 mg, and 200 mg | ➤ Subscribe | 2012-10-29 |
| ➤ Subscribe | Extended-release Tablets | 1000 mg | ➤ Subscribe | 2011-01-07 |
| ➤ Subscribe | Tablets | 7.5mg/12.5mg 15 mg/25 mg and 15 mg/12.5 mg | ➤ Subscribe | 2004-01-15 |
| ➤ Subscribe | Extended-release Transdermal Film | 1 mg/24 hr, 2 mg/24 hr, 3 mg/24 hr,4 mg/24 hr,6 mg/24 hr, and 8 mg/24 hr | ➤ Subscribe | 2013-11-26 |
International Patents for UCB INC Drugs
Supplementary Protection Certificates for UCB INC Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1033978 | 06C0025 | France | ⤷ Sign Up | PRODUCT NAME: ROTIGOTINE; REGISTRATION NO/DATE: EU/1/05/331/001 20060215 |
| 1033978 | C300236 | Netherlands | ⤷ Sign Up | PRODUCT NAME: ROTIGOTINE; REGISTRATION NO/DATE: EU/1/05/331/001-013 20060215 |
| 1452524 | CR 2016 00013 | Denmark | ⤷ Sign Up | PRODUCT NAME: BRIVARACETAM; REG. NO/DATE: EU/1/15/1073/001-022 20160118 |
| 0454511 | 99C0009 | Belgium | ⤷ Sign Up | PRODUCT NAME: IRBESARTAN / HYDROCHLOROTHIAZIDE; REGISTRATION NO/DATE: EU/1/98/086/001 19981015 |
| 1452524 | CA 2016 00013 | Denmark | ⤷ Sign Up | PRODUCT NAME: BRIVARACETAM; REG. NO/DATE: EU/1/15/1073/001-022 20160118 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.