BRIVIACT Drug Patent Profile
✉ Email this page to a colleague
When do Briviact patents expire, and what generic alternatives are available?
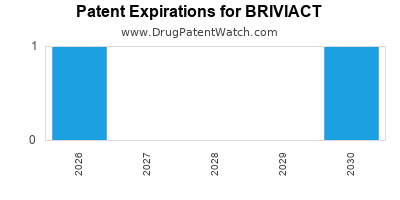
Briviact is a drug marketed by Ucb Inc and is included in three NDAs. There are two patents protecting this drug and three Paragraph IV challenges.
This drug has one hundred and seventy-three patent family members in forty-five countries.
The generic ingredient in BRIVIACT is brivaracetam. One supplier is listed for this compound. Additional details are available on the brivaracetam profile page.
DrugPatentWatch® Generic Entry Outlook for Briviact
Briviact was eligible for patent challenges on May 12, 2020.
There have been two patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There are five tentative approvals for the generic drug (brivaracetam), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for BRIVIACT?
- What are the global sales for BRIVIACT?
- What is Average Wholesale Price for BRIVIACT?
Summary for BRIVIACT
| International Patents: | 173 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 3 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 41 |
| Clinical Trials: | 15 |
| Patent Applications: | 1,094 |
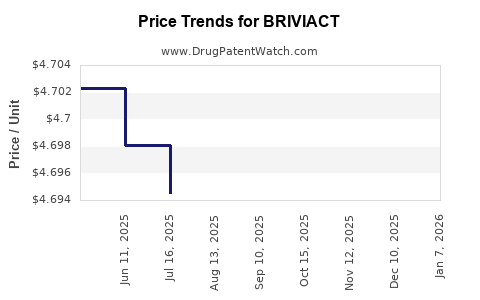
| Drug Prices: | Drug price information for BRIVIACT |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for BRIVIACT |
| What excipients (inactive ingredients) are in BRIVIACT? | BRIVIACT excipients list |
| DailyMed Link: | BRIVIACT at DailyMed |
Recent Clinical Trials for BRIVIACT
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| The Affiliated Nanjing Drum Tower Hospital of Nanjing University Medical School | PHASE1 |
| Jiangsu Chia Tai Fenghai Pharmaceutical Co., Ltd. | PHASE1 |
| University of California, Los Angeles | Phase 1/Phase 2 |
Pharmacology for BRIVIACT
| Mechanism of Action | Epoxide Hydrolase Inhibitors |
Paragraph IV (Patent) Challenges for BRIVIACT
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| BRIVIACT | Tablets | brivaracetam | 10 mg, 25 mg, 50 mg, 75 mg and 100 mg | 205836 | 7 | 2020-05-12 |
| BRIVIACT | Injection | brivaracetam | 50 mg/5 mL | 205837 | 2 | 2020-05-12 |
| BRIVIACT | Oral Solution | brivaracetam | 10 mg/mL | 205838 | 1 | 2020-05-12 |
US Patents and Regulatory Information for BRIVIACT
BRIVIACT is protected by four US patents.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ucb Inc | BRIVIACT | brivaracetam | SOLUTION;INTRAVENOUS | 205837-001 | May 12, 2016 | RX | Yes | Yes | 6,911,461 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Ucb Inc | BRIVIACT | brivaracetam | TABLET;ORAL | 205836-002 | May 12, 2016 | RX | Yes | No | 10,729,653 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Ucb Inc | BRIVIACT | brivaracetam | TABLET;ORAL | 205836-001 | May 12, 2016 | RX | Yes | No | 6,911,461 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Ucb Inc | BRIVIACT | brivaracetam | TABLET;ORAL | 205836-005 | May 12, 2016 | RX | Yes | Yes | 6,911,461 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Ucb Inc | BRIVIACT | brivaracetam | TABLET;ORAL | 205836-003 | May 12, 2016 | RX | Yes | No | 6,911,461 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Ucb Inc | BRIVIACT | brivaracetam | SOLUTION;ORAL | 205838-001 | May 12, 2016 | RX | Yes | Yes | 6,911,461 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Ucb Inc | BRIVIACT | brivaracetam | TABLET;ORAL | 205836-002 | May 12, 2016 | RX | Yes | No | 6,911,461 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for BRIVIACT
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Ucb Inc | BRIVIACT | brivaracetam | TABLET;ORAL | 205836-001 | May 12, 2016 | 8,492,416 | ⤷ Get Started Free |
| Ucb Inc | BRIVIACT | brivaracetam | TABLET;ORAL | 205836-002 | May 12, 2016 | 8,492,416 | ⤷ Get Started Free |
| Ucb Inc | BRIVIACT | brivaracetam | TABLET;ORAL | 205836-003 | May 12, 2016 | 6,784,197 | ⤷ Get Started Free |
| Ucb Inc | BRIVIACT | brivaracetam | TABLET;ORAL | 205836-004 | May 12, 2016 | 8,492,416 | ⤷ Get Started Free |
| Ucb Inc | BRIVIACT | brivaracetam | SOLUTION;INTRAVENOUS | 205837-001 | May 12, 2016 | 6,784,197 | ⤷ Get Started Free |
| Ucb Inc | BRIVIACT | brivaracetam | SOLUTION;ORAL | 205838-001 | May 12, 2016 | 6,784,197 | ⤷ Get Started Free |
| Ucb Inc | BRIVIACT | brivaracetam | TABLET;ORAL | 205836-003 | May 12, 2016 | 8,492,416 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for BRIVIACT
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| UCB Pharma SA | Briviact (in Italy: Nubriveo) | brivaracetam | EMEA/H/C/003898Briviact is indicated as adjunctive therapy in the treatment of partial-onset seizures with or without secondary generalisation in adult and adolescent patients from 16 years of age with epilepsy. | Authorised | no | no | no | 2016-01-13 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for BRIVIACT
When does loss-of-exclusivity occur for BRIVIACT?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 10215646
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 1007161
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 47395
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2292071
Estimated Expiration: ⤷ Get Started Free
Patent: 4083328
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0141006
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 15673
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 91349
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 2057
Patent: ФАРМАЦЕВТИЧЕСКИЕ КОМПОЗИЦИИ, ВКЛЮЧАЮЩИЕ БРИВАРАЦЕТАМ (PHARMACEUTICAL COMPOSITIONS COMPRISING BRIVARACETAM)
Estimated Expiration: ⤷ Get Started Free
Patent: 1101116
Patent: ФАРМАЦЕВТИЧЕСКИЕ КОМПОЗИЦИИ, ВКЛЮЧАЮЩИЕ ПРОИЗВОДНЫЕ 2-ОКСО-1-ПИРРОЛИДИНА
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 91349
Patent: Compositions pharmaceutiques comprenant des dérivés de 2-oxo-1-pyrrolidine (PHARMACEUTICAL COMPOSITIONS COMPRISING 2-OXO-1-PYRROLIDINE DERIVATIVES)
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 61988
Patent: 包含 -氧代- -吡咯烷衍生物的藥物組合物 (PHARMACEUTICAL COMPOSITIONS COMPRISING 2-OXO-1-PYRROLIDINE DERIVATIVES 2--1-)
Estimated Expiration: ⤷ Get Started Free
Patent: 98287
Patent: 包含 -氧代- -吡咯烷衍生物的藥物組合物 (PHARMACEUTICAL COMPOSITIONS COMPRISING 2-OXO-1-PYRROLIDINE DERIVATIVES 2--1-)
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 3545
Patent: תכשירי רוקחות המכילים תולדות 2-אוקסו-1-פירולידין (Pharmaceutical compositions comprising 2-oxo-1-pyrrolidine derivatives)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 76401
Estimated Expiration: ⤷ Get Started Free
Patent: 12516302
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 11007267
Patent: COMPOSICIONES FARMACEUTICAS QUE COMPRENDEN DERIVADOS DE 2-OXO-1-PIRROLIDINA. (PHARMACEUTICAL COMPOSITIONS COMPRISING 2-OXO-1-PYRROLIDINE DERIVATIVES.)
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 927
Patent: FARMACEUTSKE KOMPOZICIJE KOJE SADRZE 2-OKSO-1-PIROLIDIN DERIVATE (PHARMACEUTICAL COMPOSITIONS COMPRISING 2-OXO-1-PYRROLIDINE DERIVATIVES)
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 91349
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 91349
Estimated Expiration: ⤷ Get Started Free
San Marino
Patent: 01400155
Patent: Composizioni farmaceutiche comprendenti derivati di 2-osso-1-pirrolidina
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 554
Patent: FARMACEUTSKE KOMPOZICIJE KOJE SADRŽE 2-OKSO-1-PIROLIDIN DERIVATE (PHARMACEUTICAL COMPOSITIONS COMPRISING 2-OXO-1-PYRROLIDINE DERIVATIVES)
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 91349
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1640164
Estimated Expiration: ⤷ Get Started Free
Patent: 120008022
Patent: PHARMACEUTICAL COMPOSITIONS COMPRISING 2-OXO-1-PYRROLIDINE DERIVATIVES
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 11047
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering BRIVIACT around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 1577296 | ⤷ Get Started Free | |
| Iceland | 7919 | ⤷ Get Started Free | |
| Cyprus | 1109718 | ⤷ Get Started Free | |
| China | 1404469 | ⤷ Get Started Free | |
| South Korea | 20120008022 | PHARMACEUTICAL COMPOSITIONS COMPRISING 2-OXO-1-PYRROLIDINE DERIVATIVES | ⤷ Get Started Free |
| European Patent Office | 2391349 | Compositions pharmaceutiques comprenant des dérivés de 2-oxo-1-pyrrolidine (PHARMACEUTICAL COMPOSITIONS COMPRISING 2-OXO-1-PYRROLIDINE DERIVATIVES) | ⤷ Get Started Free |
| Yugoslavia | 63202 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for BRIVIACT
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1265862 | PA2016013 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: BRIVARACETAMAS; REGISTRATION NO/DATE: EU/1/15/1073 20160114 |
| 1452524 | 10/2016 | Austria | ⤷ Get Started Free | PRODUCT NAME: BRIVARACETAM; REGISTRATION NO/DATE: EU/1/15/1073 (MITTEILUNG) 20160118 |
| 1452524 | 16C1001 | France | ⤷ Get Started Free | PRODUCT NAME: BRIVARACETAM; REGISTRATION NO/DATE: EU/1/15/1073 20160118 |
| 1452524 | C01452524/01 | Switzerland | ⤷ Get Started Free | PRODUCT NAME: BRIVARACETAM; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 65830 06.10.2016 |
| 1452524 | 2016/011 | Ireland | ⤷ Get Started Free | PRODUCT NAME: BRIVARACETAM; REGISTRATION NO/DATE: EU/1/15/1073 20160114 |
| 2391349 | C20160006 00188 | Estonia | ⤷ Get Started Free | PRODUCT NAME: BRIVARATSETAAM;REG NO/DATE: EU/1/15/1073 18.01.2016 |
| 1452524 | 300815 | Netherlands | ⤷ Get Started Free | PRODUCT NAME: BRIVARACETAM; REGISTRATION NO/DATE: EU/1/15/1073 20160118 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for BRIVIACT
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
