NOVARTIS Company Profile
✉ Email this page to a colleague
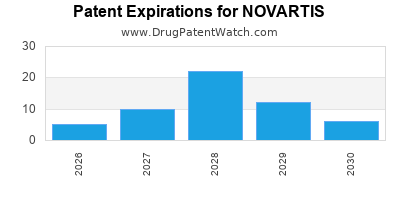
What is the competitive landscape for NOVARTIS, and when can generic versions of NOVARTIS drugs launch?
NOVARTIS has one hundred and eighty-seven approved drugs.
There are one hundred and nine US patents protecting NOVARTIS drugs.
There are two thousand seven hundred and seventy-three patent family members on NOVARTIS drugs in sixty-nine countries and five hundred and twenty-three supplementary protection certificates in nineteen countries.
Summary for NOVARTIS
| International Patents: | 2773 |
| US Patents: | 109 |
| Tradenames: | 155 |
| Ingredients: | 139 |
| NDAs: | 187 |
| Drug Master File Entries: | 1 |
| Patent Litigation for NOVARTIS: | See patent lawsuits for NOVARTIS |
| PTAB Cases with NOVARTIS as patent owner: | See PTAB cases with NOVARTIS as patent owner |
Drugs and US Patents for NOVARTIS
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novartis | PROMACTA | eltrombopag olamine | TABLET;ORAL | 022291-001 | Nov 20, 2008 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | |||||
| Novartis Pharms Corp | ENTRESTO | sacubitril; valsartan | TABLET;ORAL | 207620-002 | Jul 7, 2015 | RX | Yes | No | 8,101,659*PED | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Novartis | DEXACIDIN | dexamethasone; neomycin sulfate; polymyxin b sulfate | OINTMENT;OPHTHALMIC | 062566-001 | Feb 22, 1985 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for NOVARTIS
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Novartis | EXFORGE HCT | amlodipine besylate; hydrochlorothiazide; valsartan | TABLET;ORAL | 022314-004 | Apr 30, 2009 | 6,294,197*PED | ⤷ Sign Up |
| Novartis | DIOVAN HCT | hydrochlorothiazide; valsartan | TABLET;ORAL | 020818-002 | Mar 6, 1998 | 6,294,197*PED | ⤷ Sign Up |
| Novartis | GLEEVEC | imatinib mesylate | CAPSULE;ORAL | 021335-002 | May 10, 2001 | RE43932*PED | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for NOVARTIS drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 10 mg | ➤ Subscribe | 2014-06-18 |
| ➤ Subscribe | Tablets | 2.5 mg | ➤ Subscribe | 2006-03-02 |
| ➤ Subscribe | Capsules | 0.5 mg | ➤ Subscribe | 2014-09-22 |
| ➤ Subscribe | Tablets | 80 mg/12.5 mg, 160 mg/12.5 mg and 160 mg/25 mg | ➤ Subscribe | 2005-12-02 |
| ➤ Subscribe | Capsules | 400 mg | ➤ Subscribe | 2014-01-24 |
| ➤ Subscribe | Tablets | 180 mg | ➤ Subscribe | 2015-10-23 |
| ➤ Subscribe | Tablets | 5 mg/160 mg | ➤ Subscribe | 2007-10-22 |
| ➤ Subscribe | Capsules | 150 mg and 200 mg | ➤ Subscribe | 2013-01-29 |
| ➤ Subscribe | Tablets | 10 mg/160 mg | ➤ Subscribe | 2007-10-01 |
| ➤ Subscribe | Injection | 4 mg/100 mg, 100 mL vial | ➤ Subscribe | 2012-01-31 |
| ➤ Subscribe | Tablets | 5 mg/12.5 mg/160 mg, 5 mg/25 mg/160 mg, 10 mg/25 mg/160 mg and 10 mg/25 mg/320 m | ➤ Subscribe | 2009-09-14 |
| ➤ Subscribe | Nasal Spray | 0.665 mg/ Spray | ➤ Subscribe | 2009-06-29 |
| ➤ Subscribe | Tablets | 125 mg, 250 mg and 500 mg | ➤ Subscribe | 2004-12-28 |
| ➤ Subscribe | Oral Suspension | 300 mg/5 mL | ➤ Subscribe | 2006-12-26 |
| ➤ Subscribe | Tablets | 12.5 mg and 25 mg | ➤ Subscribe | 2014-02-04 |
| ➤ Subscribe | Delayed-release Tablets | 180 mg | ➤ Subscribe | 2009-06-04 |
| ➤ Subscribe | Tablets | 250 mg | ➤ Subscribe | 2011-03-14 |
| ➤ Subscribe | Capsules | 1.5 mg, 3 mg, 4.5 mg and 6 mg | ➤ Subscribe | 2004-04-21 |
| ➤ Subscribe | Tablets | 125 mg, 250 mg, and 500 mg | ➤ Subscribe | 2011-10-28 |
| ➤ Subscribe | Tablets for Oral Suspension | 2 mg, 3 mg and 5 mg | ➤ Subscribe | 2016-12-30 |
| ➤ Subscribe | Transdermal System Extended-release | 13.3 mg/24 hr | ➤ Subscribe | 2013-01-22 |
| ➤ Subscribe | Tablets | 2.5 mg, 5 mg, and 7.5 mg | ➤ Subscribe | 2014-12-10 |
| ➤ Subscribe | Tablets | 90 mg and 360 mg | ➤ Subscribe | 2015-10-19 |
| ➤ Subscribe | Tablets | 100 mg and 400 mg | ➤ Subscribe | 2007-03-12 |
| ➤ Subscribe | Tablets | 320 mg/12.5 mg and 320 mg/25 mg | ➤ Subscribe | 2007-02-07 |
| ➤ Subscribe | Capsules | 20 mg and 40 mg | ➤ Subscribe | 2008-06-04 |
| ➤ Subscribe | Tablets | 180 mg | ➤ Subscribe | 2016-04-28 |
| ➤ Subscribe | Tablets | 5 mg/320 mg | ➤ Subscribe | 2007-11-26 |
| ➤ Subscribe | Injection | 0.8 mg (base) /mL | ➤ Subscribe | 2008-06-11 |
| ➤ Subscribe | Tablets | 10 mg/320 mg | ➤ Subscribe | 2007-11-09 |
| ➤ Subscribe | Ophthalmic Solution | 0.003% | ➤ Subscribe | 2015-12-30 |
| ➤ Subscribe | Tablets | 10 mg/12.5 mg/160 mg | ➤ Subscribe | 2009-10-22 |
| ➤ Subscribe | Tablets | 150 mg, 300 mg and 600 mg | ➤ Subscribe | 2006-05-05 |
| ➤ Subscribe | Tablets | 50 mg and 75 mg | ➤ Subscribe | 2014-01-07 |
| ➤ Subscribe | Delayed-release Tablets | 360 mg | ➤ Subscribe | 2009-02-02 |
| ➤ Subscribe | Tablets | 60 mg and 120 mg | ➤ Subscribe | 2004-12-22 |
| ➤ Subscribe | Tablets | 40 mg, 80 mg,160 mg | ➤ Subscribe | 2004-12-28 |
| ➤ Subscribe | Extended-release Tablets | 100 mg | ➤ Subscribe | 2005-12-30 |
| ➤ Subscribe | Tablets | 0.25 mg, 0.5 mg, and 0.75 mg | ➤ Subscribe | 2013-09-30 |
| ➤ Subscribe | Oral Solution | 2 mg/mL | ➤ Subscribe | 2004-11-05 |
International Patents for NOVARTIS Drugs
Supplementary Protection Certificates for NOVARTIS Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2340828 | C202130003 | Spain | ⤷ Sign Up | PRODUCT NAME: SACUBITRILO/VALSARTAN, COMO COMPLEJO SALINO DE SACUBITRILO, VALSARTAN Y SODIO, ES DECIR (((S)-N-VALERIL-N-((2'-(1H-TETRAZOL-5-IL)-BIFENIL-4-IL)-METIL)-VALINA) / ESTER ETILICO DEL ACIDO ((2R,4S)-5-BIFENIL-4-IL-4-(3-CARBOXI-PROPIONIL AMINO)-2-METIL-PENTANOICO)) NA3 - X H2O, EN EL QUE X ES 0 A 3; NATIONAL AUTHORISATION NUMBER: EU/1/15/1058; DATE OF AUTHORISATION: 20151119; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/15/1058; DATE OF FIRST AUTHORISATION IN EEA: 20151119 |
| 1343782 | 28/2010 | Austria | ⤷ Sign Up | PRODUCT NAME: PAZOPANIB, GEGEBENENFALLS IN FORM EINES PHARMAZEUTISCH ANNEHMBAREN SALZES ODER SOLVATS DAVON; REGISTRATION NO/DATE: EU/1/10/628/001 - EU/1/10/628/004 20100614 |
| 0503785 | C300375 | Netherlands | ⤷ Sign Up | PRODUCT NAME: COMBINATIE VAN OLMESARTAN MEDOXOMIL, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT EN AMLODIPINEBESILAAT; REGISTRATION NO/DATE: RVG100984, RVG100986-87, RVG100989-91, RVG100993-95 20080819 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.