BOEHRINGER INGELHEIM Company Profile
✉ Email this page to a colleague
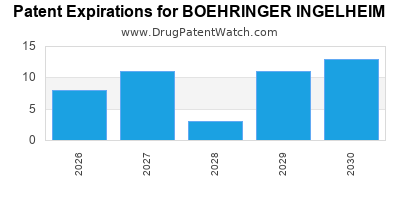
What is the competitive landscape for BOEHRINGER INGELHEIM, and when can generic versions of BOEHRINGER INGELHEIM drugs launch?
BOEHRINGER INGELHEIM has forty-eight approved drugs.
There are seventy US patents protecting BOEHRINGER INGELHEIM drugs.
There are one thousand eight hundred and twenty-nine patent family members on BOEHRINGER INGELHEIM drugs in sixty-three countries and two hundred and seventeen supplementary protection certificates in nineteen countries.
Summary for BOEHRINGER INGELHEIM
| International Patents: | 1829 |
| US Patents: | 70 |
| Tradenames: | 37 |
| Ingredients: | 29 |
| NDAs: | 48 |
| Patent Litigation for BOEHRINGER INGELHEIM: | See patent lawsuits for BOEHRINGER INGELHEIM |
| PTAB Cases with BOEHRINGER INGELHEIM as petitioner: | See PTAB cases with BOEHRINGER INGELHEIM as petitioner |
Drugs and US Patents for BOEHRINGER INGELHEIM
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim | TRIJARDY XR | empagliflozin; linagliptin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 212614-001 | Jan 27, 2020 | RX | Yes | No | 8,551,957*PED | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Boehringer Ingelheim | ALUPENT | metaproterenol sulfate | SOLUTION;INHALATION | 018761-001 | Jun 30, 1983 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | |||||
| Boehringer Ingelheim | TRADJENTA | linagliptin | TABLET;ORAL | 201280-001 | May 2, 2011 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for BOEHRINGER INGELHEIM
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim | SPIRIVA RESPIMAT | tiotropium bromide | SPRAY, METERED;INHALATION | 021936-002 | Sep 15, 2015 | 6,176,442 | ⤷ Sign Up |
| Boehringer Ingelheim | ATROVENT | ipratropium bromide | SPRAY, METERED;NASAL | 020393-001 | Oct 20, 1995 | 4,385,048 | ⤷ Sign Up |
| Boehringer Ingelheim | COMBIVENT RESPIMAT | albuterol sulfate; ipratropium bromide | SPRAY, METERED;INHALATION | 021747-001 | Oct 7, 2011 | 6,846,413 | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for BOEHRINGER INGELHEIM drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 10 mg/5 mg and 25 mg/5 mg | ➤ Subscribe | 2018-08-01 |
| ➤ Subscribe | Tablets | 2.5 mg/500 mg, 2.5 mg/850 mg, 2.5 mg/1000 mg | ➤ Subscribe | 2015-05-04 |
| ➤ Subscribe | Tablets | 80 mg/12.5 mg and 40 mg/12.5 mg | ➤ Subscribe | 2008-12-31 |
| ➤ Subscribe | Extended-release Capsules | 25 mg and 200 mg | ➤ Subscribe | 2007-02-01 |
| ➤ Subscribe | Tablets | 0.125 mg, 0.5 mg, 1 mg and 1.5 mg | ➤ Subscribe | 2005-06-24 |
| ➤ Subscribe | Capsules | eq. to 75 mg base and 150 mg base | ➤ Subscribe | 2014-10-20 |
| ➤ Subscribe | Tablets | 0.75 mg | ➤ Subscribe | 2008-07-31 |
| ➤ Subscribe | Inhalation Powder Capsules | 18 mcg | ➤ Subscribe | 2018-05-11 |
| ➤ Subscribe | Tablets | 5 mg/500 mg5 mg/1000 mg12.5 mg/500 mg12.5 mg/1000 mg | ➤ Subscribe | 2018-08-01 |
| ➤ Subscribe | Extended-release Tablets | 0.375 mg, 0.75 mg, 1.5 mg, 3 mg and 4.5 mg | ➤ Subscribe | 2010-06-01 |
| ➤ Subscribe | Extended-release Tablets | 5 mg/1000 mg10 mg/1000 mg12.5 mg/1000 mg25 mg/1000 mg | ➤ Subscribe | 2018-08-01 |
| ➤ Subscribe | Tablets | 20 mg, 30 mg and 40 mg | ➤ Subscribe | 2017-07-12 |
| ➤ Subscribe | Tablets | 10 mg and 25 mg | ➤ Subscribe | 2018-08-01 |
| ➤ Subscribe | Tablets | 20 mg, 40 mg and 80 mg | ➤ Subscribe | 2006-12-26 |
| ➤ Subscribe | Tablets | 80 mg/25 mg | ➤ Subscribe | 2009-02-27 |
| ➤ Subscribe | Capsules | 100 mg and 150 mg | ➤ Subscribe | 2018-10-15 |
| ➤ Subscribe | Tablets | 0.25 mg | ➤ Subscribe | 2005-05-27 |
| ➤ Subscribe | Capsules | eq. to 110 mg base | ➤ Subscribe | 2015-12-15 |
| ➤ Subscribe | Oral Suspension | 7.5 mg/5 mL | ➤ Subscribe | 2009-12-17 |
| ➤ Subscribe | Extended-releaseTablets | 2.5 mg/1000 mg 5 mg/1000 mg | ➤ Subscribe | 2018-03-28 |
| ➤ Subscribe | Tablets | 5 mg | ➤ Subscribe | 2015-05-04 |
| ➤ Subscribe | Extended-release Tablets | 2.25 mg and 3.75 mg | ➤ Subscribe | 2011-07-26 |
| ➤ Subscribe | Extended-release Tablets | 400 mg | ➤ Subscribe | 2013-06-21 |
International Patents for BOEHRINGER INGELHEIM Drugs
Supplementary Protection Certificates for BOEHRINGER INGELHEIM Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1830843 | 2015C/038 | Belgium | ⤷ Sign Up | PRODUCT NAME: NINTEDANIB, OU UN TAUTOMERE DE CELUI-CI, DES MELANGES DE CEUX-CI OU UN SEL DE CELUI-CI, SPECIFIQUEMENTNINTEDANIB SOUS FORME D'ESILATE; AUTHORISATION NUMBER AND DATE: EU/1/14/979/001 20150119 |
| 1224170 | CR 2015 00019 | Denmark | ⤷ Sign Up | PRODUCT NAME: NINTEDANIB, TAUTOMERERNE OG SALTENE DERAF, SAERLIGT NINTEDANIB OG FYSIOLOGISK ACCEPTABLE SALTE DERAF, SPECIFIKT NINTEDANIB ESILAT; REG. NO/DATE: EU/1/14/954/001-004 20141121 |
| 1485094 | CA 2012 00054 | Denmark | ⤷ Sign Up | PRODUCT NAME: DABIGATRAN-ETEXILAT OG SALTENE DERAF; SAERLIGT DABIGATRAN-ETEXILAT-MESYLAT; REG. NO/DATE: EU/1/08/442/009-014 20080318 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.