TRIJARDY XR Drug Patent Profile
✉ Email this page to a colleague
When do Trijardy Xr patents expire, and when can generic versions of Trijardy Xr launch?
Trijardy Xr is a drug marketed by Boehringer Ingelheim and is included in one NDA. There are seventeen patents protecting this drug and one Paragraph IV challenge.
This drug has five hundred and six patent family members in forty-eight countries.
The generic ingredient in TRIJARDY XR is empagliflozin; linagliptin; metformin hydrochloride. There are twenty-two drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the empagliflozin; linagliptin; metformin hydrochloride profile page.
DrugPatentWatch® Generic Entry Outlook for Trijardy Xr
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be May 21, 2030. This may change due to patent challenges or generic licensing.
There have been fifty-five patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for TRIJARDY XR?
- What are the global sales for TRIJARDY XR?
- What is Average Wholesale Price for TRIJARDY XR?
Summary for TRIJARDY XR
| International Patents: | 506 |
| US Patents: | 17 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Clinical Trials: | 1 |
| Drug Prices: | Drug price information for TRIJARDY XR |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for TRIJARDY XR |
| What excipients (inactive ingredients) are in TRIJARDY XR? | TRIJARDY XR excipients list |
| DailyMed Link: | TRIJARDY XR at DailyMed |
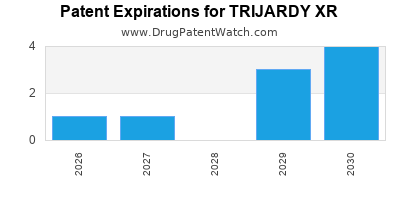
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for TRIJARDY XR
Generic Entry Date for TRIJARDY XR*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET, EXTENDED RELEASE;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for TRIJARDY XR
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Humanis Saglk Anonim Sirketi | PHASE1 |
Pharmacology for TRIJARDY XR
Paragraph IV (Patent) Challenges for TRIJARDY XR
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| TRIJARDY XR | Extended-release Tablets | empagliflozin; linagliptin; metformin hydrochloride | 5 mg/2.5 mg/1 g, 10 mg/5 mg/1 g, 12.5 mg/5 mg/1 g, 25 mg/5 mg/1 g | 212614 | 1 | 2020-05-26 |
US Patents and Regulatory Information for TRIJARDY XR
TRIJARDY XR is protected by eighteen US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of TRIJARDY XR is ⤷ Get Started Free.
This potential generic entry date is based on patent 9,155,705.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim | TRIJARDY XR | empagliflozin; linagliptin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 212614-002 | Jan 27, 2020 | RX | Yes | No | 10,258,637*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Boehringer Ingelheim | TRIJARDY XR | empagliflozin; linagliptin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 212614-003 | Jan 27, 2020 | RX | Yes | No | 9,949,998*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Boehringer Ingelheim | TRIJARDY XR | empagliflozin; linagliptin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 212614-004 | Jan 27, 2020 | RX | Yes | Yes | 9,415,016 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for TRIJARDY XR
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim | TRIJARDY XR | empagliflozin; linagliptin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 212614-003 | Jan 27, 2020 | 8,178,541 | ⤷ Get Started Free |
| Boehringer Ingelheim | TRIJARDY XR | empagliflozin; linagliptin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 212614-001 | Jan 27, 2020 | 6,488,962 | ⤷ Get Started Free |
| Boehringer Ingelheim | TRIJARDY XR | empagliflozin; linagliptin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 212614-002 | Jan 27, 2020 | 8,119,648 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for TRIJARDY XR
When does loss-of-exclusivity occur for TRIJARDY XR?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 1175
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 09232043
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0911273
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 20450
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 09000809
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1983073
Estimated Expiration: ⤷ Get Started Free
Patent: 3083672
Estimated Expiration: ⤷ Get Started Free
Patent: 6215190
Estimated Expiration: ⤷ Get Started Free
Patent: 3648422
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 51277
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 85410
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 10010489
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 9395
Estimated Expiration: ⤷ Get Started Free
Patent: 8435
Estimated Expiration: ⤷ Get Started Free
Patent: 1001577
Estimated Expiration: ⤷ Get Started Free
Patent: 1300121
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 85410
Estimated Expiration: ⤷ Get Started Free
Patent: 53403
Estimated Expiration: ⤷ Get Started Free
Patent: 44374
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 49485
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 41649
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 88428
Estimated Expiration: ⤷ Get Started Free
Patent: 22068
Estimated Expiration: ⤷ Get Started Free
Patent: 11516456
Estimated Expiration: ⤷ Get Started Free
Patent: 13237707
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 1232
Estimated Expiration: ⤷ Get Started Free
Patent: 10010819
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 200
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 7747
Estimated Expiration: ⤷ Get Started Free
Patent: 9580
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 091730
Estimated Expiration: ⤷ Get Started Free
Patent: 140960
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 85410
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1005664
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1611314
Estimated Expiration: ⤷ Get Started Free
Patent: 1775942
Estimated Expiration: ⤷ Get Started Free
Patent: 110005690
Estimated Expiration: ⤷ Get Started Free
Patent: 160042174
Estimated Expiration: ⤷ Get Started Free
Patent: 170056021
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 96124
Estimated Expiration: ⤷ Get Started Free
Patent: 12839
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 27816
Estimated Expiration: ⤷ Get Started Free
Patent: 0946534
Estimated Expiration: ⤷ Get Started Free
Patent: 1509941
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 10000431
Estimated Expiration: ⤷ Get Started Free
Turkey
Patent: 1818886
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 4136
Estimated Expiration: ⤷ Get Started Free
Uruguay
Patent: 747
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering TRIJARDY XR around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Spain | 2702174 | ⤷ Get Started Free | |
| European Patent Office | 4144374 | INHIBITEUR DE DPP-IV COMBINÉ À UN AUTRE AGENT ANTIDIABÉTIQUE, COMPRIMÉS COMPRENANT DE TELLES FORMULATIONS, LEUR UTILISATION ET LEUR PROCÉDÉ DE PRÉPARATION (DPP-IV INHIBITOR COMBINED WITH A FURTHER ANTIDIABETIC AGENT, TABLETS COMPRISING SUCH FORMULATIONS, THEIR USE AND PROCESS FOR THEIR PREPARATION) | ⤷ Get Started Free |
| Taiwan | 201433316 | Use of DPP IV inhibitors | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for TRIJARDY XR
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1532149 | 8/2012 | Austria | ⤷ Get Started Free | PRODUCT NAME: 8-(3-AMINOPIPERIDIN-1-YL)-7-BUT-2-INYL-3-METHYL-1-(4-METHYLCHINAZOLIN-2-YLMETHYL)-3,7-DIHYDROPURIN-2,6-DION UND DESSEN SALZE, INSBES. LINAGLIPTIN; REGISTRATION NO/DATE: EU/1/11/707/001-011 (MITTEILUNG) 20110830 |
| 2187879 | 132017000050485 | Italy | ⤷ Get Started Free | PRODUCT NAME: COMBINAZIONE DI EMPAGLIFLOZIN E LINAGLIPTIN O UN SUO SALE FARMACEUTICAMENTE ACCETTABILE(GLYXAMBI); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/16/1146, 20161115 |
| 2187879 | 122017000024 | Germany | ⤷ Get Started Free | PRODUCT NAME: EMPAGLIFLOZIN MIT LINAGLIPTIN ODER EINEM PHARMAZEUTISCH AKZEPTABLEN SALZ HIERVON; REGISTRATION NO/DATE: EU/1/16/1146/001-018 20161111 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for TRIJARDY XR
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
