Alnylam Pharms Inc Company Profile
✉ Email this page to a colleague
What is the competitive landscape for ALNYLAM PHARMS INC, and what generic alternatives to ALNYLAM PHARMS INC drugs are available?
ALNYLAM PHARMS INC has four approved drugs.
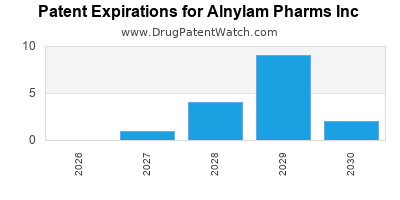
There are forty-four US patents protecting ALNYLAM PHARMS INC drugs.
There are seven hundred and seventy patent family members on ALNYLAM PHARMS INC drugs in fifty-four countries and seventy-five supplementary protection certificates in seventeen countries.
Summary for Alnylam Pharms Inc
| International Patents: | 770 |
| US Patents: | 44 |
| Tradenames: | 4 |
| Ingredients: | 4 |
| NDAs: | 4 |
Drugs and US Patents for Alnylam Pharms Inc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alnylam Pharms Inc | AMVUTTRA | vutrisiran sodium | SOLUTION;SUBCUTANEOUS | 215515-001 | Jun 13, 2022 | RX | Yes | Yes | 10,806,791 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Alnylam Pharms Inc | GIVLAARI | givosiran sodium | SOLUTION;SUBCUTANEOUS | 212194-001 | Nov 20, 2019 | RX | Yes | Yes | 10,131,907 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Alnylam Pharms Inc | OXLUMO | lumasiran sodium | SOLUTION;SUBCUTANEOUS | 214103-001 | Nov 23, 2020 | RX | Yes | Yes | 10,487,330 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Alnylam Pharms Inc | GIVLAARI | givosiran sodium | SOLUTION;SUBCUTANEOUS | 212194-001 | Nov 20, 2019 | RX | Yes | Yes | 10,273,477 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Alnylam Pharms Inc | ONPATTRO | patisiran sodium | SOLUTION;INTRAVENOUS | 210922-001 | Aug 10, 2018 | RX | Yes | Yes | 8,642,076 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Alnylam Pharms Inc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Alnylam Pharms Inc | ONPATTRO | patisiran sodium | SOLUTION;INTRAVENOUS | 210922-001 | Aug 10, 2018 | 8,895,718 | ⤷ Try a Trial |
| Alnylam Pharms Inc | ONPATTRO | patisiran sodium | SOLUTION;INTRAVENOUS | 210922-001 | Aug 10, 2018 | 8,552,171 | ⤷ Try a Trial |
| Alnylam Pharms Inc | ONPATTRO | patisiran sodium | SOLUTION;INTRAVENOUS | 210922-001 | Aug 10, 2018 | 8,362,231 | ⤷ Try a Trial |
| Alnylam Pharms Inc | GIVLAARI | givosiran sodium | SOLUTION;SUBCUTANEOUS | 212194-001 | Nov 20, 2019 | 9,708,615 | ⤷ Try a Trial |
| Alnylam Pharms Inc | ONPATTRO | patisiran sodium | SOLUTION;INTRAVENOUS | 210922-001 | Aug 10, 2018 | 8,778,902 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for Alnylam Pharms Inc Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| South Korea | 20170099832 | ⤷ Try a Trial |
| Japan | 5843914 | ⤷ Try a Trial |
| Portugal | 3431076 | ⤷ Try a Trial |
| Hong Kong | 1200191 | ⤷ Try a Trial |
| Slovenia | 3185957 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Alnylam Pharms Inc Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3052628 | 132020000000106 | Italy | ⤷ Try a Trial | PRODUCT NAME: GIVOSIRAN O UN SUO SALE FARMACEUTICAMENTE ACCETTABILE(GIVLAARI); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/20/1428, 20200304 |
| 3581654 | LUC00218 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: LUMASIRAN; AUTHORISATION NUMBER AND DATE: EU/1/20/1496 20201123 |
| 3581654 | 21C1044 | France | ⤷ Try a Trial | PRODUCT NAME: LUMASIRAN; REGISTRATION NO/DATE: EU/1/20/1496 20201123 |
| 3052628 | PA2020527,C3052628 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: GIVOSIRANAS ARBA JO FARMACINIU POZIURIU PRIIMTINA DRUSKA; REGISTRATION NO/DATE: EU/1/20/1428 20200302 |
| 3204015 | C202230012 | Spain | ⤷ Try a Trial | PRODUCT NAME: LUMASIRAN, OPCIONALMENTE EN FORMA DE UNA SAL; NATIONAL AUTHORISATION NUMBER: EU/1/20/1496; DATE OF AUTHORISATION: 20201119; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/20/1496; DATE OF FIRST AUTHORISATION IN EEA: 20201119 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.