VORICONAZOLE Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Voriconazole, and what generic alternatives are available?
Voriconazole is a drug marketed by Amneal Pharms, Hetero Labs Ltd V, Novel Labs Inc, Rising, Zhejiang Poly Pharm, Almaject, Aspiro, Eugia Pharma, Gland, Hikma, Meitheal, Pharmobedient, Sandoz Inc, Slate Run Pharma, UBI, Zydus Pharms, Ajanta Pharma Ltd, Aurobindo Pharma Ltd, Cadila, Chartwell Rx, Epic Pharma Llc, Glenmark Pharms Ltd, Mylan Pharms Inc, Prinston Inc, and Teva Pharms. and is included in twenty-seven NDAs.
The generic ingredient in VORICONAZOLE is voriconazole. There are fourteen drug master file entries for this compound. Thirty-one suppliers are listed for this compound. Additional details are available on the voriconazole profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Voriconazole
A generic version of VORICONAZOLE was approved as voriconazole by MYLAN PHARMS INC on April 22nd, 2010.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for VORICONAZOLE?
- What are the global sales for VORICONAZOLE?
- What is Average Wholesale Price for VORICONAZOLE?
Summary for VORICONAZOLE
| US Patents: | 0 |
| Applicants: | 25 |
| NDAs: | 27 |
| Finished Product Suppliers / Packagers: | 30 |
| Raw Ingredient (Bulk) Api Vendors: | 127 |
| Clinical Trials: | 160 |
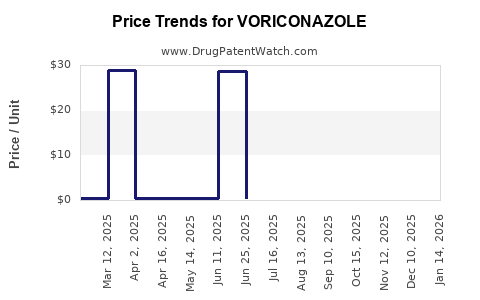
| Drug Prices: | Drug price information for VORICONAZOLE |
| What excipients (inactive ingredients) are in VORICONAZOLE? | VORICONAZOLE excipients list |
| DailyMed Link: | VORICONAZOLE at DailyMed |
Recent Clinical Trials for VORICONAZOLE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Elion Therapeutics, Inc. | PHASE2 |
| Melinta Therapeutics | PHASE3 |
| Fernanda P Silveira, MD, MS | PHASE3 |
Pharmacology for VORICONAZOLE
| Drug Class | Azole Antifungal |
| Mechanism of Action | Cytochrome P450 2C19 Inhibitors Cytochrome P450 2C9 Inhibitors Cytochrome P450 3A4 Inhibitors |
Medical Subject Heading (MeSH) Categories for VORICONAZOLE
Anatomical Therapeutic Chemical (ATC) Classes for VORICONAZOLE
Paragraph IV (Patent) Challenges for VORICONAZOLE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| VFEND | Oral Suspension | voriconazole | 40 mg/mL | 021630 | 1 | 2010-10-08 |
| VFEND | For Injection | voriconazole | 200 mg/vial | 021267 | 1 | 2008-09-12 |
| VFEND | Tablets | voriconazole | 50 mg and 200 mg | 021266 | 1 | 2008-04-14 |
US Patents and Regulatory Information for VORICONAZOLE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prinston Inc | VORICONAZOLE | voriconazole | TABLET;ORAL | 206654-002 | Aug 8, 2016 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Glenmark Pharms Ltd | VORICONAZOLE | voriconazole | TABLET;ORAL | 203503-002 | Sep 2, 2015 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Rising | VORICONAZOLE | voriconazole | TABLET;ORAL | 206762-001 | May 24, 2016 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Mylan Pharms Inc | VORICONAZOLE | voriconazole | TABLET;ORAL | 090547-001 | Apr 22, 2010 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for VORICONAZOLE
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Pfizer Europe MA EEIG | Vfend | voriconazole | EMEA/H/C/000387Voriconazole, is a broad spectrum, triazole antifungal agent and is indicated in adults and children aged 2 years and above as follows:treatment of invasive aspergillosis;treatment of in candidaemianon-neutropenic patients;treatment of fluconazole-resistant serious invasive Candida infections (including C. krusei);Treatment of serious fungal infections caused by Scedosporium spp. and Fusarium spp.Vfend should be administered primarily to patients with progressive, possibly life-threatening infections.Prophylaxis of invasive fungal infections in high risk allogeneic hematopoietic stem cell transplant (HSCT) recipients. | Authorised | no | no | no | 2002-03-19 | |
| Accord Healthcare S.L.U. | Voriconazole Accord | voriconazole | EMEA/H/C/002669Voriconazole is a broad-spectrum, triazole antifungal agent and is indicated in adults and children aged two years and above as follows:treatment of invasive aspergillosis;treatment of candidaemia in non-neutropenic patients;treatment of fluconazole-resistant serious invasive Candida infections (including C. krusei);Treatment of serious fungal infections caused by Scedosporium spp. and Fusarium spp.Voriconazole Accord should be administered primarily to patients with progressive, possibly life-threatening infections. | Authorised | yes | no | no | 2013-05-16 | |
| Hikma Farmaceutica (Portugal) S.A. | Voriconazole Hikma (previously Voriconazole Hospira) | voriconazole | EMEA/H/C/003737Voriconazole is a broad spectrum, triazole antifungal agent and is indicated in adults and children aged 2 years and above as follows:treatment of invasive aspergillosis;treatment of candidaemia in non-neutropenic patients;treatment of fluconazole-resistant serious invasive Candida infections (including C. krusei);treatment of serious fungal infections caused by Scedosporium spp. and Fusarium spp.Voriconazole should be administered primarily to patients with progressive, possibly life-threatening infections.Prophylaxis of invasive fungal infections in high risk allogeneic hematopoietic stem cell transplant (HSCT)recipients. | Authorised | yes | no | no | 2015-05-27 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
Market Dynamics and Financial Trajectory for Voriconazole
More… ↓
