VAGIFEM Drug Patent Profile
✉ Email this page to a colleague
When do Vagifem patents expire, and when can generic versions of Vagifem launch?
Vagifem is a drug marketed by Novo Nordisk Inc and is included in one NDA.
The generic ingredient in VAGIFEM is estradiol. There are seventy-five drug master file entries for this compound. Forty-six suppliers are listed for this compound. Additional details are available on the estradiol profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Vagifem
A generic version of VAGIFEM was approved as estradiol by BARR LABS INC on October 22nd, 1997.
Summary for VAGIFEM
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 109 |
| Clinical Trials: | 12 |
| Patent Applications: | 6,715 |
| Formulation / Manufacturing: | see details |
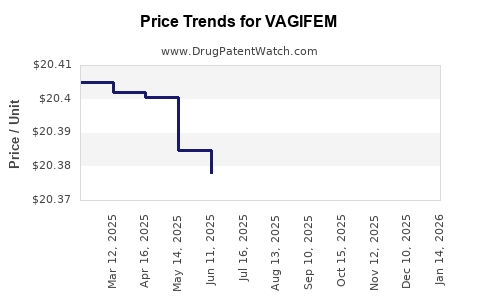
| Drug Prices: | Drug price information for VAGIFEM |
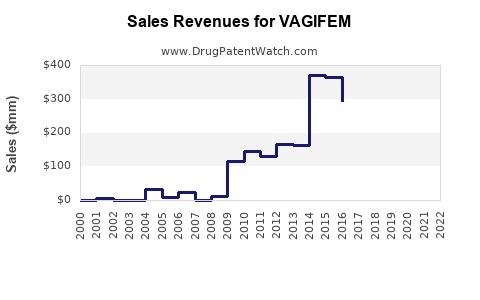
| Drug Sales Revenues: | Drug sales revenues for VAGIFEM |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for VAGIFEM |
| What excipients (inactive ingredients) are in VAGIFEM? | VAGIFEM excipients list |
| DailyMed Link: | VAGIFEM at DailyMed |
Recent Clinical Trials for VAGIFEM
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Angelica Lindén Hirschberg | Phase 4 |
| University Hospitals Cleveland Medical Center | Phase 4 |
| University of California, San Diego | Phase 3 |
Pharmacology for VAGIFEM
| Drug Class | Estrogen |
| Mechanism of Action | Estrogen Receptor Agonists |
Anatomical Therapeutic Chemical (ATC) Classes for VAGIFEM
US Patents and Regulatory Information for VAGIFEM
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novo Nordisk Inc | VAGIFEM | estradiol | TABLET;VAGINAL | 020908-002 | Nov 25, 2009 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Novo Nordisk Inc | VAGIFEM | estradiol | TABLET;VAGINAL | 020908-001 | Mar 26, 1999 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for VAGIFEM
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Novo Nordisk Inc | VAGIFEM | estradiol | TABLET;VAGINAL | 020908-002 | Nov 25, 2009 | ⤷ Try a Trial | ⤷ Try a Trial |
| Novo Nordisk Inc | VAGIFEM | estradiol | TABLET;VAGINAL | 020908-002 | Nov 25, 2009 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for VAGIFEM
See the table below for patents covering VAGIFEM around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 4851057 | ⤷ Try a Trial | |
| Germany | 10179223 | ⤷ Try a Trial | |
| Canada | 2258310 | INSTRUMENT POUR L'INTRODUCTION D'UN SUPPOSITOIRE (INSTRUMENT FOR INSERTING A SUPPOSITORY) | ⤷ Try a Trial |
| Japan | 2010189409 | USE OF OESTROGEN IN MANUFACTURE OF COMPOSITION CONTAINING OESTROGEN FOR TREATMENT OF ATROPHIC VAGINITIS | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 0247692 | ⤷ Try a Trial | |
| Japan | 2004515531 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for VAGIFEM
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1453521 | 132016000025143 | Italy | ⤷ Try a Trial | PRODUCT NAME: LEVONORGESTREL ED ETINILESTRADIOLO(SEASONIQUE); AUTHORISATION NUMBER(S) AND DATE(S): 17/0017/15-S, 20150211;042139016, 20150414 |
| 1453521 | 300814 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: LEVONORGESTREL EN ETHINYLESTRADIOL; NATIONAL REGISTRATION NO/DATE: RVG 117453 20151211; FIRST REGISTRATION: SK 17/0017/15-S 20150211 |
| 0770388 | PA2009004 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOLI VALERAS + DIENOGESTUM; NAT. REGISTRATION NO/DATE: LT/1/09/1512/001, 2009 04 06 LT/1/09/1512/002, 2009 04 06 LT/1/09/1512/003 20090406; FIRST REGISTRATION: BE 327792 20081103 |
| 0136011 | 99C0003 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOL AND NORETHISTERONE; FIRST REGISTRATION NO/DATE: 403 IS 106 F3 19980928; FIRST REGISTRATION: SE 14007 19980306 |
| 2782584 | 301153 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: COMPOSITION CONTAINING BOTH ESTRADIOL (17SS-ESTRADIOL), OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, HYDRATE OR SOLVATE THEREOF (INCLUDING IN HEMIHYDRATE FORM), AND PROGESTERONE; NATIONAL REGISTRATION NO/DATE: RVG 125821 20210611; FIRST REGISTRATION: BE BE582231 20210406 |
| 1214076 | 49/2008 | Austria | ⤷ Try a Trial | PRODUCT NAME: WIRKSTOFFKOMBINATION VON ETHINYLESTRADIOL UND DROSPIRENON; REGISTRATION NO/DATE: 1-27586 20080612 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


