Last updated: July 27, 2025
Introduction
Temozolomide, marketed primarily under the brand name Temodar, is an oral alkylating chemotherapy agent used predominantly for the treatment of glioblastoma multiforme (GBM) and other malignant brain tumors. Since its initial approval, temozolomide has established itself as a cornerstone in neuro-oncology, catalyzing shifts in treatment paradigms and influencing the pharmaceutical landscape. This analysis explores the market dynamics influencing temozolomide’s growth and evaluates its projected financial trajectory amid evolving clinical, regulatory, and competitive environments.
Market Overview and Current Landscape
Historical Market Context
Approved by the U.S. Food and Drug Administration (FDA) in 2005, temozolomide revolutionized the management of gliomas by offering an oral, well-tolerated, and effective chemotherapeutic option. Its integration into standard-of-care protocols, specifically concurrent with radiotherapy for GBM, propelled rapid adoption across developed markets, predominantly North America, Europe, and parts of Asia-Pacific.
Initial market uptake was fueled by limited alternatives for aggressive brain tumors and the drug’s demonstrated survival benefits. The patent held by Schering-Plough (later acquired by Merck) provided exclusivity through the early 2020s, creating a high-margin environment and incentivizing continued investment.
Current Market Size and Share
As of 2023, the global market for temozolomide is valued at approximately USD 600 million, with North America accounting for nearly 50% due to high prevalence and advanced healthcare infrastructure. Europe and Asia-Pacific contribute the remaining share, with emerging markets showing increased access and usage.
The drug’s revenue is driven predominantly by indications for glioblastoma and anaplastic astrocytoma. Off-label uses, such as certain neuroendocrine tumors, contribute marginally. The market is characterized by high drug adherence rates owing to its oral formulation, but faced with patent expirations and the advent of biosimilars, future revenue streams are under pressure.
Market Dynamics Shaping Future Trajectory
Patent Expiration and Biosimilar Entry
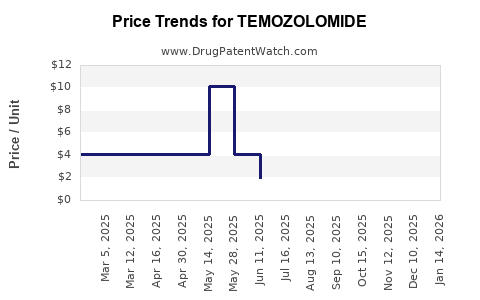
The primary driver of anticipated revenue decline is the impending expiration of key patents, expected between 2023 and 2026. The patent cliff has catalyzed the entry of biosimilar and generic versions, reducing prices by up to 40%. Major pharmaceutical companies are preparing for this transition, with some low-cost entrants already available in select markets, intensifying competition and eroding profit margins.
Regulatory Developments and Off-Label Approvals
Regulatory bodies are increasingly scrutinizing off-label claims, emphasizing evidence-based indications. While temozolomide's primary indications remain approved, ongoing clinical trials investigating its efficacy in other cancers, such as melanoma and certain leukemias, may expand or restrict its scope, influencing sales accordingly. Additionally, the approval of combination therapies incorporating temozolomide continues to evolve, potentially affecting its market positioning.
Emerging Therapies and Treatment Paradigm Shifts
Innovations in neuro-oncology threaten to displace monotherapy with combination or immunotherapy-based regimens. Trials such as CheckMate 548 and those investigating tumor-treating fields (TTFields) aim for survival improvements, possibly reducing reliance on temozolomide in standard protocols. The advent of targeted therapies and immuno-oncology agents, like nivolumab and pembrolizumab, pose competitive threats and may impact sales volumes.
Patient Demographics and Disease Prevalence
The rising incidence of gliomas, driven by aging populations and increased diagnostic capabilities, sustains demand. According to the Central Brain Tumor Registry of the United States (CBTRUS), GBM incidence is approximately 3.2 cases per 100,000 annually, with demographic shifts potentially increasing it further.
Pricing and Reimbursement Policies
Healthcare payer pressure for cost containment affects drug pricing. Managed care organizations and government payers are negotiating for discounts and formulary exclusions, especially post-patent expiry. Value-based pricing models and risk-sharing agreements are emerging, influencing revenue streams.
Financial Trajectory and Outlook
Short-Term Predictions (2023-2025)
In the immediate future, revenues are expected to plateau or decline modestly due to patent expiration and the rise of generics. However, existing inventory and continued use in approved indications will sustain stable cash flows for a period. The shift towards biosimilar adoption might trigger price reductions, diminishing profit margins substantially.
Medium- to Long-Term Outlook (2026-2030)
Post-patent, the market will witness heightened competition with generic and biosimilar versions capturing significant market share. Price erosion could reach 50-60%, impacting top-line revenues unless offset by volume increases or new indications.
Simultaneously, orphan drug designations and expanded indications, such as recurrent gliomas or combination treatments under development, could provide alternative revenue streams. Merck and other pharmaceutical stakeholders are investing in clinical programs to extend the drug’s lifecycle through innovative delivery systems and companion diagnostics.
Projected Revenue Trends
Industry analysts forecast a decline in global temozolomide sales by approximately 35-45% over the next five years, contingent on patent expiries and biosimilar market penetration. Nonetheless, the drug's role as a standard-of-care in newly diagnosed GBM ensures a baseline demand in markets where it remains reimbursed and preferred.
Impact of Pricing Strategies and Market Penetration
Adapting to market pressures, pharmaceutical companies are deploying volume-based strategies, negotiating rebates, and exploring tiered pricing models in lower-income regions. These approaches aim to preserve market share amid competition, albeit with reduced margins.
Additionally, licensing agreements and strategic alliances with local manufacturers facilitate access and mitigate supply chain risks.
Strategic Considerations for Stakeholders
-
Pharmaceutical Manufacturers: Invest in pipeline augmentation, including combination therapies and novel delivery platforms, to prolong market relevance.
-
Investors and Business Analysts: Focus on developing alternative revenue models, emphasizing pipeline expansion, and monitoring biosimilar trends.
-
Healthcare Providers: Optimize prescribing practices by considering cost-effectiveness, especially as generics become more prevalent.
-
Regulatory Bodies: Streamline approval pathways for innovative formulations and combinatory regimens that could impact the traditional role of temozolomide.
Key Takeaways
-
Market Maturity and Competition: Temozolomide’s patent expiration triggers significant brand erosion, opening markets for biosimilars and generics, which substantially reduce revenues and profit margins.
-
Revenue Declines Forecasted: Industry projections indicate a 35-45% sales decline over the next five years, driven by increased competition and pricing pressures.
-
Pipeline and Indication Expansion: Ongoing clinical trials and new combination regimes are crucial for maintaining relevance and creating alternative revenue streams.
-
Pricing and Access Strategies: Sophisticated pricing models, including value-based agreements, are critical for optimizing market presence in price-sensitive regions.
-
Market Adaptability: Stakeholders must pivot toward innovation, licensing agreements, and broader indications to sustain financial viability.
FAQs
1. When is the patent for temozolomide expiring, and what does that mean for its market?
The primary patents for temozolomide are set to expire between 2023 and 2026. This expiration allows biosimilar and generic manufacturers to enter the market, intensifying competition, reducing prices, and potentially decreasing brand-name sales.
2. How are biosimilars expected to impact temozolomide’s revenue?
Biosimilars are projected to capture a substantial market share, leading to significant price reductions—potentially up to 50-60%. This shift is expected to diminish the profitability of innovator companies and reshape the market landscape.
3. What emerging therapies could replace or complement temozolomide?
Immuno-oncology agents like PD-1 inhibitors, tumor-treating fields, and targeted therapies are under clinical investigation. Successful development of these options could reduce reliance on temozolomide as a first-line treatment.
4. Are there ongoing efforts to expand temozolomide’s indications?
Yes. Clinical trials are exploring its efficacy in other tumors, such as metastatic melanoma and certain leukemias, with potential for indication expansion, which could mitigate revenue loss from generics.
5. How can pharmaceutical companies mitigate revenue decline post-patent expiry?
Strategies include developing combination therapies, innovating delivery methods, securing new indications, engaging in licensing and partnerships, and implementing value-based pricing models.
Sources:
[1] CBTRUS. Central Brain Tumor Registry of the United States, 2021.
[2] IMS Health Data. Global Oncology Market Analysis, 2022.
[3] U.S. FDA. Temozolomide Approval and Regulatory Information, 2005.
[4] Industry Reports. Biosimilar Impact on Cancer Therapies, 2022.
[5] ClinicalTrials.gov. Ongoing trials involving Temozolomide, 2023.