QBRELIS Drug Patent Profile
✉ Email this page to a colleague
When do Qbrelis patents expire, and when can generic versions of Qbrelis launch?
Qbrelis is a drug marketed by Azurity and is included in one NDA. There are nine patents protecting this drug and one Paragraph IV challenge.
This drug has ten patent family members in eight countries.
The generic ingredient in QBRELIS is lisinopril. There are twenty-one drug master file entries for this compound. Forty-five suppliers are listed for this compound. Additional details are available on the lisinopril profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Qbrelis
A generic version of QBRELIS was approved as lisinopril by ASCENT PHARMS INC on July 1st, 2002.
Summary for QBRELIS
| International Patents: | 10 |
| US Patents: | 9 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 82 |
| Clinical Trials: | 2 |
| Formulation / Manufacturing: | see details |
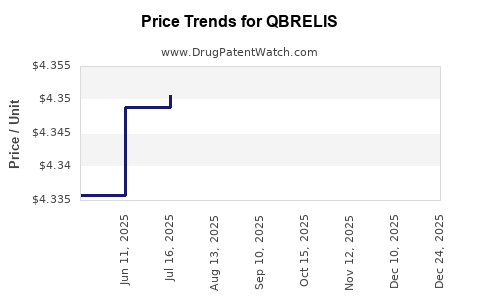
| Drug Prices: | Drug price information for QBRELIS |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for QBRELIS |
| What excipients (inactive ingredients) are in QBRELIS? | QBRELIS excipients list |
| DailyMed Link: | QBRELIS at DailyMed |
Recent Clinical Trials for QBRELIS
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| National Cancer Institute (NCI) | Phase 2 |
| St. Jude Children's Research Hospital | Phase 2 |
| Mayo Clinic | Phase 2 |
Pharmacology for QBRELIS
| Drug Class | Angiotensin Converting Enzyme Inhibitor |
| Mechanism of Action | Angiotensin-converting Enzyme Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for QBRELIS
Paragraph IV (Patent) Challenges for QBRELIS
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| QBRELIS | Oral Solution | lisinopril | 1 mg/mL | 208401 | 1 | 2019-10-24 |
US Patents and Regulatory Information for QBRELIS
QBRELIS is protected by twenty-seven US patents.
Patents protecting QBRELIS
Lisinopril formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ACUTE MYOCARDIAL INFARCTION
Lisinopril formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF MYOCARDIAL INFARCTION
Lisinopril formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: REDUCTION OF MORTALITY IN ACUTE MYOCARDIAL INFARCTION
Lisinopril formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATMENT OF HEART FAILURE
Lisinopril formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF HEART FAILURE
Lisinopril formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF HYPERTENSION
Lisinopril formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING HYPERTENSION
Lisinopril formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Lisinopril formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF MYOCARDIAL INFARCTION
Lisinopril formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING HYPERTENSION
Lisinopril formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF HEART FAILURE
Lisinopril formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATMENT OF HEART FAILURE
Lisinopril formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: REDUCTION OF MORTALITY IN ACUTE MYOCARDIAL INFARCTION
Lisinopril formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF HYPERTENSION
Lisinopril formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ACUTE MYOCARDIAL INFARCTION
Lisinopril formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Lisinopril formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Lisinopril formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF HYPERTENSION
Lisinopril formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING HYPERTENSION
Lisinopril formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF HEART FAILURE
Lisinopril formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF MYOCARDIAL INFARCTION
Lisinopril formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: REDUCTION OF MORTALITY IN ACUTE MYOCARDIAL INFARCTION
Lisinopril formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ACUTE MYOCARDIAL INFARCTION
Lisinopril formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATMENT OF HEART FAILURE
Lisinopril formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Azurity | QBRELIS | lisinopril | SOLUTION;ORAL | 208401-001 | Jul 29, 2016 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Azurity | QBRELIS | lisinopril | SOLUTION;ORAL | 208401-001 | Jul 29, 2016 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Azurity | QBRELIS | lisinopril | SOLUTION;ORAL | 208401-001 | Jul 29, 2016 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Azurity | QBRELIS | lisinopril | SOLUTION;ORAL | 208401-001 | Jul 29, 2016 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Azurity | QBRELIS | lisinopril | SOLUTION;ORAL | 208401-001 | Jul 29, 2016 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Azurity | QBRELIS | lisinopril | SOLUTION;ORAL | 208401-001 | Jul 29, 2016 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Azurity | QBRELIS | lisinopril | SOLUTION;ORAL | 208401-001 | Jul 29, 2016 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for QBRELIS
See the table below for patents covering QBRELIS around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 3960156 | FORMULATIONS DE LISINOPRIL (LISINOPRIL FORMULATIONS) | ⤷ Try a Trial |
| China | 112972370 | 赖诺普利制剂 (LISINOPRIL FORMULATIONS) | ⤷ Try a Trial |
| Hong Kong | 1258803 | 賴諾普利製劑 (LISINOPRIL FORMULATIONS) | ⤷ Try a Trial |
| European Patent Office | 3368012 | FORMULATIONS DE LISINOPRIL (LISINOPRIL FORMULATIONS) | ⤷ Try a Trial |
| China | 108472252 | 赖诺普利制剂 (LISINOPRIL FORMULATIONS) | ⤷ Try a Trial |
| Spain | 2886869 | ⤷ Try a Trial | |
| World Intellectual Property Organization (WIPO) | 2017075368 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |


