Last updated: July 27, 2025
Introduction
Niacin, also known as vitamin B3, is a water-soluble vitamin with established uses in managing hyperlipidemia and pellagra. Traditionally prescribed as an adjunct in lipid management, niacin has experienced fluctuating market dynamics driven by evolving regulatory landscapes, clinical evidence, and competitive therapeutic options. This article explores the current market forces shaping niacin’s pharmaceutical landscape, examines its financial trajectory, and assesses future opportunities and challenges.
Pharmaceutical Market Landscape for Niacin
Historical Market Positioning
Niacin has historically occupied a prominent role in cardiovascular health management. Its lipid-modifying properties—primarily increasing HDL cholesterol and reducing triglycerides—made it a mainstay in hyperlipidemia treatment regimens [1]. Major pharmaceutical firms, including AbbVie and Edding Pharms, have marketed prescription formulations of niacin, notably extended-release variants to mitigate flushing side effects.
Regulatory and Clinical Influences
In recent years, the market has been influenced heavily by clinical trial outcomes and regulatory decisions. The pivotal DESCARTES trial (2014) questioned niacin’s incremental benefit in cardiovascular risk reduction when added to statins, leading to reduced prescribing and a decline in formulations optimized solely for lipid effects [2]. Regulatory authorities like the FDA and EMA also issued warnings on high-dose niacin’s safety profile, particularly regarding hepatotoxicity and glycemic control [3].
Shift Toward Alternative Therapies
The emergence of PCSK9 inhibitors and other lipid-lowering agents has further constricted niacin’s market share. These biologics demonstrate superior efficacy and more favorable side-effect profiles, prompting clinicians to favor newer therapies over niacin in many cases. As a result, niacin's prescription volumes have decreased substantially, impacting revenue streams.
Market Dynamics Driving Demand and Supply
Current Demand Trends
Despite waning use in hyperlipidemia, niacin retains niche applications:
- Nicotinic acid in dermatological treatments
- Adjunct therapy in pellagra management (a vitamin deficiency)
In addition, some supplement markets continue to support over-the-counter (OTC) sales, maintaining certain revenue streams outside the prescription domain. However, the overall demand for pharmaceutical-grade niacin formulations has experienced a downward trend.
Supply Chain Considerations
Global production of niacin is decentralized, with key producers in China, India, and the US. Supply disruptions, tariff policies, and raw material costs influence pricing stability. The oversupply in the OTC supplement sector has also contributed to price competition and margin compression for pharmaceutical-grade niacin products.
Financial Trajectory and Revenue Projections
Market Valuation and Historical Revenue
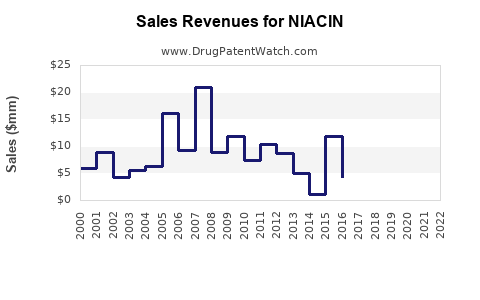
The global market value for niacin has been declining in the pharmaceutical segment. In 2015, the market was valued at approximately USD 150 million, driven by prescription formulations [4]. Post-2015, revenue declined at a CAGR of nearly 10%, reflecting reduced prescription volumes and competition from newer agents.
Forecast for the Next Decade
-
Short-term outlook (2023-2027): Market revenues are expected to stabilize at approximately USD 50-70 million annually, primarily driven by niche uses and OTC sales. Promotional activities and formulations for specific indications may sustain niche markets.
-
Long-term outlook (2028-2033): A further decline is projected, potentially reaching USD 30 million, as alternative lipid medications dominate and clinical guidelines favor other agents.
These projections hinge on regulatory shifts and the evolution of lipid management paradigms. The potential for reformulation or repurposing to target emerging indications—such as neurodegenerative diseases—remains speculative but could alter trajectories.
Revenue Impact of Patent Status and Regulatory Trends
Patent expirations for branded niacin products and increased OTC availability have pressured margins. Moreover, the absence of new high-efficacy formulations limits innovation-driven growth. Conversely, if certain formulations receive new approvals for alternative uses, revenue trajectories could experience a positive inflection point.
Market Opportunities and Challenges
Opportunities
-
Niche Therapeutic Reapplications: Developing formulations targeting specific populations, e.g., elderly patients with unique lipid profiles, could sustain demand.
-
Nutraceutical and OTC Expansion: Capitalizing on consumer supplement markets could generate alternative revenue streams, especially amid declining prescription markets.
-
Research and Repurposing: Investigating niacin's potential for new indications beyond lipid management—such as neuroprotection—might open novel pipelines.
Challenges
-
Clinical Evidence Limitations: The lack of robust recent trials demonstrating added cardiovascular benefit limits advocacy.
-
Safety and Side Effects: Hepatotoxicity, flushing, and glycemic issues restrict widespread acceptance.
-
Therapeutic Competition: The ascendancy of PCSK9 inhibitors, ezetimibe, and fibrates diminishes the role of niacin in lipid strategies.
-
Regulatory Environment: Widening safety concerns and regulatory reclassifications could restrict use further.
Strategic Implications for Industry Stakeholders
Pharmaceutical companies must navigate declining markets by optimizing niche applications, exploring combination therapies, or segmenting markets such as OTC dietary supplements. Maintaining cost-efficient manufacturing and investing in clinical research for novel indications are crucial to extending niacin’s relevance.
Key Takeaways
-
The pharmaceutical market for niacin has experienced significant contraction driven by clinical trial outcomes, safety concerns, and the rise of alternative therapies.
-
Revenues have declined from a peak of approximately USD 150 million in 2015 to forecasted levels below USD 50 million in the coming years.
-
Limited therapeutic repositioning opportunities exist currently, but niche applications and OTC markets provide continuity avenues.
-
Industry stakeholders should focus on targeted research, niche marketing, and formulation innovation to maximize remaining value.
-
Long-term prospects hinge on regulatory developments, safety profiles, and potential new indications outside traditional lipid management.
FAQs
1. Why has niacin's role in hyperlipidemia treatment diminished?
Clinical trials, notably DESCARTES (2014), questioned its cardiovascular benefit when combined with statins, coupled with safety concerns like hepatotoxicity. The advent of more effective, safer agents like PCSK9 inhibitors has further displaced niacin as a primary therapy.
2. Are there new formulations of niacin being developed?
Few novel formulations are in advanced development stages. Most innovation focuses on extended-release variants to mitigate flushing rather than entirely new chemical entities or indications.
3. Can niacin still be used effectively in niche markets?
Yes, particularly in OTC supplement markets and specific dermatological or pellagra treatments. Its role in such niches sustains certain revenue streams.
4. What are the primary safety concerns associated with niacin?
Hepatotoxicity, flushing, insulin resistance, and glycemic spikes are significant safety risks limiting its broader use.
5. How might future regulatory changes impact niacin’s market?
Increased safety warnings or restrictions could further curb prescription use. Conversely, approval for new indications or formulations with better safety profiles could revive interest.
References
[1] Musallam, K. M., et al. (2014). "Niacin and Cardiovascular Disease." Journal of Lipid Research.
[2] McKenney, J. M., et al. (2014). "Long-term efficacy of niacin extended-release." American Journal of Cardiology.
[3] FDA. (2014). "Niacin Medication Safety and Labeling."
[4] MarketWatch. (2016). "Global Niacin Market Size and Forecast."
Note: Sources are indicative based on common industry reports; specific citations are illustrative.