NEVIRAPINE Drug Patent Profile
✉ Email this page to a colleague
When do Nevirapine patents expire, and what generic alternatives are available?
Nevirapine is a drug marketed by Aurobindo, Cipla, Alvogen, Apotex, Aurobindo Pharma, Macleods Pharms Ltd, Mylan, Pharmobedient, Sandoz, Tech Organized, Apotex Inc, Hetero Labs Ltd Iii, Micro Labs Ltd, Mylan Pharms Inc, Prinston Inc, and Strides Pharma. and is included in twenty-three NDAs.
The generic ingredient in NEVIRAPINE is nevirapine. There are twenty drug master file entries for this compound. Six suppliers are listed for this compound. Additional details are available on the nevirapine profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Nevirapine
A generic version of NEVIRAPINE was approved as nevirapine by AUROBINDO on May 22nd, 2012.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for NEVIRAPINE?
- What are the global sales for NEVIRAPINE?
- What is Average Wholesale Price for NEVIRAPINE?
Summary for NEVIRAPINE
| US Patents: | 0 |
| Applicants: | 16 |
| NDAs: | 23 |
| Finished Product Suppliers / Packagers: | 6 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 222 |
| Patent Applications: | 5,705 |
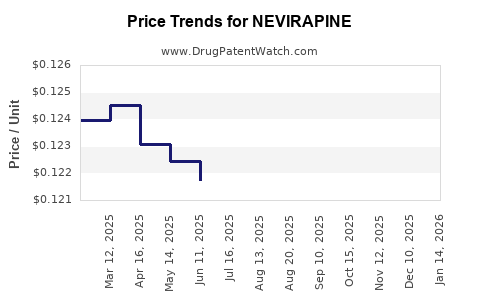
| Drug Prices: | Drug price information for NEVIRAPINE |
| DailyMed Link: | NEVIRAPINE at DailyMed |
Recent Clinical Trials for NEVIRAPINE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| ANRS, Emerging Infectious Diseases | PHASE2 |
| Centre de Recherches et d'Etude sur la Pathologie Tropicale et le Sida | PHASE2 |
| Organization providing support methodology coordination (Institut Pierre Louis d'Epidmiologie et de Sant Publique) | PHASE2 |
Pharmacology for NEVIRAPINE
Medical Subject Heading (MeSH) Categories for NEVIRAPINE
Anatomical Therapeutic Chemical (ATC) Classes for NEVIRAPINE
Paragraph IV (Patent) Challenges for NEVIRAPINE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| NEVIRAPINE | Injection | nevirapine | 5 mg/vial | 203411 | 1 | 2016-12-21 |
| VIRAMUNE XR | Extended-release Tablets | nevirapine | 400 mg | 201152 | 3 | 2013-06-21 |
US Patents and Regulatory Information for NEVIRAPINE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aurobindo | NEVIRAPINE | nevirapine | SUSPENSION;ORAL | 077702-001 | May 22, 2012 | AA | RX | No | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Hetero Labs Ltd Iii | NEVIRAPINE | nevirapine | TABLET;ORAL | 078584-001 | May 22, 2012 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Strides Pharma | NEVIRAPINE | nevirapine | TABLET;ORAL | 078195-001 | May 22, 2012 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for NEVIRAPINE
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim International GmbH | Viramune | nevirapine | EMEA/H/C/000183Tablets and oral suspensionViramune is indicated in combination with other antiretroviral medicinal products for the treatment of HIV-1-infected adults, adolescents, and children of any age.Most of the experience with Viramune is in combination with nucleoside reverse-transcriptase inhibitors (NRTIs). The choice of a subsequent therapy after Viramune should be based on clinical experience and resistance testing.50- and 100-mg prolonged-release tabletsViramune is indicated in combination with other antiretroviral medicinal products for the treatment of HIV-1-infected adolescents and children three years and above and able to swallow tablets.Prolonged-release tablets are not suitable for the 14-day lead-in phase for patients starting nevirapine. Other nevirapine formulations, such as immediate-release tablets or oral suspension should be used.Most of the experience with Viramune is in combination with nucleoside reverse-transcriptase inhibitors (NRTIs). The choice of a subsequent therapy after Viramune should be based on clinical experience and resistance testing.400-mg prolonged-release tabletsViramune is indicated in combination with other antiretroviral medicinal products for the treatment of HIV-1-infected adults, adolescents and children three years and above and able to swallow tablets.Prolonged-release tablets are not suitable for the 14-day lead-in phase for patients starting nevirapine. Other nevirapine formulations, such as immediate-release tablets or oral suspension should be used.Most of the experience with Viramune is in combination with nucleoside reverse-transcriptase inhibitors (NRTIs). The choice of a subsequent therapy after Viramune should be based on clinical experience and resistance testing. | Authorised | no | no | no | 1998-02-04 | |
| Teva B.V. | Nevirapine Teva | nevirapine | EMEA/H/C/001119Nevirapine Teva is indicated in combination with other anti-retroviral medicinal products for the treatment of HIV 1 infected adults, adolescents, and children of any age.Most of the experience with nevirapine is in combination with nucleoside reverse transcriptase inhibitors (NRTIs). The choice of a subsequent therapy after nevirapine should be based on clinical experience and resistance testing. | Withdrawn | yes | no | no | 2009-11-30 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
Market Dynamics and Financial Trajectory for Nevirapine
More… ↓
