ISOSORBIDE Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Isosorbide, and what generic alternatives are available?
Isosorbide is a drug marketed by Impax Labs Inc, Sun Pharm Inds Inc, Ani Pharms, Hikma Intl Pharms, Par Pharm, Par Pharm Inc, Rubicon, Sandoz, Sun Pharm Industries, Superpharm, Watson Labs, Zydus, Watson Labs Teva, I3 Pharms, Riconpharma Llc, Accord Hlthcare, Actavis Elizabeth, Alkermes Gainesville, Aurobindo Pharma, Chartwell Molecular, Dexcel Ltd, Ivax Sub Teva Pharms, Shandong, Skyepharma Ag, Strides Pharma, Torrent Pharms, Zydus Hlthcare, Zydus Pharms, and Hikma Pharms. and is included in fifty-six NDAs.
The generic ingredient in ISOSORBIDE is isosorbide mononitrate. There are thirty-seven drug master file entries for this compound. Twenty-two suppliers are listed for this compound. Additional details are available on the isosorbide mononitrate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Isosorbide
A generic version of ISOSORBIDE was approved as isosorbide mononitrate by CHARTWELL MOLECULAR on October 30th, 1998.
Summary for ISOSORBIDE
| US Patents: | 0 |
| Applicants: | 29 |
| NDAs: | 56 |
| Formulation / Manufacturing: | see details |
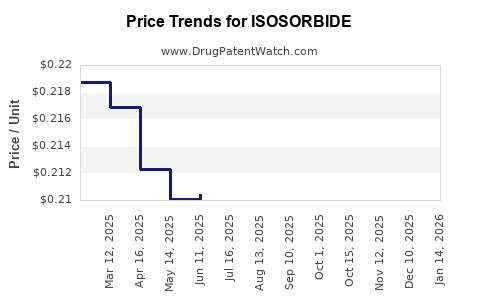
| Drug Prices: | Drug price information for ISOSORBIDE |
| DailyMed Link: | ISOSORBIDE at DailyMed |
Recent Clinical Trials for ISOSORBIDE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Copenhagen University Hospital, Hvidovre | Phase 4 |
| Johannes Grand | Phase 4 |
| Bispebjerg Hospital | Phase 4 |
Medical Subject Heading (MeSH) Categories for ISOSORBIDE
US Patents and Regulatory Information for ISOSORBIDE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strides Pharma | ISOSORBIDE MONONITRATE | isosorbide mononitrate | TABLET, EXTENDED RELEASE;ORAL | 090598-001 | Aug 11, 2010 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Watson Labs | ISOSORBIDE DINITRATE | isosorbide dinitrate | TABLET;SUBLINGUAL | 086033-001 | Feb 26, 1988 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Zydus | ISOSORBIDE DINITRATE | isosorbide dinitrate | TABLET;ORAL | 213057-004 | Nov 20, 2019 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Ani Pharms | ISOSORBIDE MONONITRATE | isosorbide mononitrate | TABLET;ORAL | 075147-001 | Nov 27, 1998 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Actavis Elizabeth | ISOSORBIDE MONONITRATE | isosorbide mononitrate | TABLET, EXTENDED RELEASE;ORAL | 075306-002 | Dec 31, 1998 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |


