GRANISETRON Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Granisetron, and when can generic versions of Granisetron launch?
Granisetron is a drug marketed by Am Regent, Amneal, Baxter Hlthcare Corp, Bionpharma, Dr Reddys, Epic Pharma Llc, Eugia Pharma, Fresenius Kabi Usa, Hikma, Hikma Farmaceutica, Mylan Asi, Mylan Labs Ltd, Rising, Sandoz, Sandoz Inc, Teva Pharms Usa, Wockhardt Usa, Yung Shin Pharm, Apotex Inc, Aurobindo Pharma Usa, Barr, Chartwell Molecular, Dr Reddys Labs Ltd, Natco Pharma, Orbion Pharms, Taro, and Teva Pharms. and is included in forty-six NDAs.
The generic ingredient in GRANISETRON is granisetron hydrochloride. There are twenty-six drug master file entries for this compound. Seven suppliers are listed for this compound. Additional details are available on the granisetron hydrochloride profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Granisetron
A generic version of GRANISETRON was approved as granisetron hydrochloride by AMNEAL on December 31st, 2007.
Summary for GRANISETRON
| US Patents: | 0 |
| Applicants: | 27 |
| NDAs: | 46 |
| Formulation / Manufacturing: | see details |
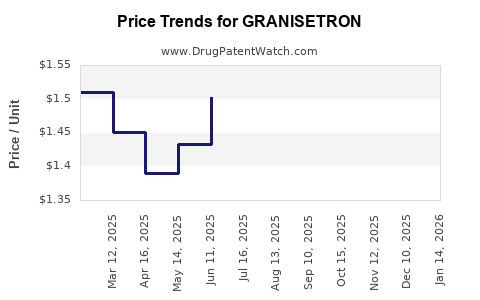
| Drug Prices: | Drug price information for GRANISETRON |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for GRANISETRON |
| DailyMed Link: | GRANISETRON at DailyMed |
Recent Clinical Trials for GRANISETRON
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Second Affiliated Hospital, School of Medicine, Zhejiang University | Phase 4 |
| Guangxi Medical University | Phase 3 |
| Heron Therapeutics | Phase 4 |
Medical Subject Heading (MeSH) Categories for GRANISETRON
Paragraph IV (Patent) Challenges for GRANISETRON
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| SANCUSO | Transdermal System | granisetron | 3.1 mg/24 hrs | 022198 | 1 | 2015-10-09 |
US Patents and Regulatory Information for GRANISETRON
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yung Shin Pharm | GRANISETRON HYDROCHLORIDE | granisetron hydrochloride | INJECTABLE;INJECTION | 202647-001 | Mar 6, 2020 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sandoz | GRANISETRON HYDROCHLORIDE | granisetron hydrochloride | INJECTABLE;INJECTION | 078808-001 | Apr 29, 2008 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Epic Pharma Llc | GRANISETRON HYDROCHLORIDE | granisetron hydrochloride | TABLET;ORAL | 078260-001 | Dec 31, 2007 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for GRANISETRON
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Kyowa Kirin Holdings B.V. | Sancuso | granisetron | EMEA/H/C/002296 Prevention of nausea and vomiting in patients receiving moderately or highly emetogenic chemotherapy, with or without cisplatin, for up to five consecutive days.Sancuso may be used in patients receiving their first chemotherapy regimen or in patients who have previously received chemotherapy. |
Authorised | no | no | no | 2012-04-20 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |


