FYCOMPA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Fycompa, and what generic alternatives are available?
Fycompa is a drug marketed by Catalyst Pharms and is included in two NDAs. There are two patents protecting this drug and two Paragraph IV challenges.
This drug has one hundred and one patent family members in thirty-three countries.
The generic ingredient in FYCOMPA is perampanel. There are five drug master file entries for this compound. Two suppliers are listed for this compound. Additional details are available on the perampanel profile page.
DrugPatentWatch® Generic Entry Outlook for Fycompa
Fycompa was eligible for patent challenges on October 22, 2016.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be July 1, 2026. This may change due to patent challenges or generic licensing.
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There are three tentative approvals for the generic drug (perampanel), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
Summary for FYCOMPA
| International Patents: | 101 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 59 |
| Clinical Trials: | 22 |
| Patent Applications: | 372 |
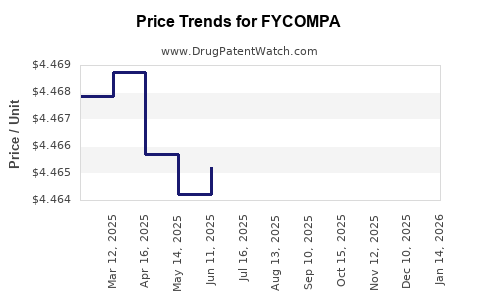
| Drug Prices: | Drug price information for FYCOMPA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for FYCOMPA |
| What excipients (inactive ingredients) are in FYCOMPA? | FYCOMPA excipients list |
| DailyMed Link: | FYCOMPA at DailyMed |
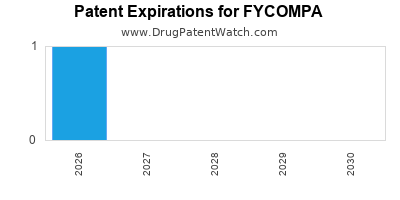
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for FYCOMPA
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for FYCOMPA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Wayne State University | Phase 4 |
| Eisai Korea Inc. | Phase 4 |
| University of Florida | Phase 4 |
Pharmacology for FYCOMPA
Anatomical Therapeutic Chemical (ATC) Classes for FYCOMPA
Paragraph IV (Patent) Challenges for FYCOMPA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| FYCOMPA | Oral Suspension | perampanel | 0.5 mg/mL | 208277 | 1 | 2022-12-20 |
| FYCOMPA | Tablets | perampanel | 2 mg, 4 mg, 6 mg, 8 mg, 10 mg and 12 mg | 202834 | 2 | 2016-10-24 |
US Patents and Regulatory Information for FYCOMPA
FYCOMPA is protected by six US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of FYCOMPA is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting FYCOMPA
1,2-dihydropyridine compounds, process for preparation of the same and use thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF PARTIAL-ONSET SEIZURES WITH OR WITHOUT SECONDARILY GENERALIZED SEIZURES IN PATIENTS WITH EPILEPSY 4 YEARS OF AGE AND OLDER
1,2-dihydropyridine compounds, process for preparation of the same and use thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF PARTIAL-ONSET SEIZURES WITH OR WITHOUT SECONDARILY GENERALIZED SEIZURES IN PATIENTS WITH EPILEPSY
1,2-dihydropyridine compounds, process for preparation of the same and use thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF PRIMARY GENERALIZED TONIC-CLONIC SEIZURES AS ADJUNCTIVE THERAPY IN PATIENTS WITH EPILEPSY
1,2-dihydropyridine compounds, process for preparation of the same and use thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF EPILEPSY
1,2-dihydropyridine compounds, process for preparation of the same and use thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF PRIMARY GENERALIZED TONIC-CLONIC SEIZURES AS ADJUNCTIVE THERAPY IN PATIENTS WITH EPILEPSY 12 YEARS OF AGE AND OLDER
Method for producing 1, 2-dihydropyridine-2-one compound
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Catalyst Pharms | FYCOMPA | perampanel | SUSPENSION;ORAL | 208277-001 | Apr 29, 2016 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Catalyst Pharms | FYCOMPA | perampanel | TABLET;ORAL | 202834-002 | Oct 22, 2012 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Catalyst Pharms | FYCOMPA | perampanel | TABLET;ORAL | 202834-003 | Oct 22, 2012 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for FYCOMPA
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Eisai GmbH | Fycompa | perampanel | EMEA/H/C/002434 Fycompa is indicated for the adjunctive treatment of partial-onset seizures with or without secondarily generalised seizures in adult and adolescent patients from 12 years of age with epilepsy.Fycompa is indicated for the adjunctive treatment of primary generalised tonic-clonic seizures in adult and adolescent patients from 12 years of age with idiopathic generalised epilepsy. |
Authorised | no | no | no | 2012-07-23 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for FYCOMPA
When does loss-of-exclusivity occur for FYCOMPA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 05258378
Estimated Expiration: ⤷ Sign Up
Patent: 05258385
Estimated Expiration: ⤷ Sign Up
Austria
Patent: 47405
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 0510254
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 61829
Estimated Expiration: ⤷ Sign Up
Patent: 70177
Estimated Expiration: ⤷ Sign Up
China
Patent: 42443
Estimated Expiration: ⤷ Sign Up
Patent: 84890
Estimated Expiration: ⤷ Sign Up
Patent: 1544599
Estimated Expiration: ⤷ Sign Up
Patent: 1914057
Estimated Expiration: ⤷ Sign Up
Patent: 2382047
Estimated Expiration: ⤷ Sign Up
Patent: 4402807
Estimated Expiration: ⤷ Sign Up
Patent: 4402808
Estimated Expiration: ⤷ Sign Up
Croatia
Patent: 0130363
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 14313
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 64361
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 64361
Estimated Expiration: ⤷ Sign Up
Patent: 72450
Estimated Expiration: ⤷ Sign Up
Patent: 86771
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 02127
Estimated Expiration: ⤷ Sign Up
Patent: 04999
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 0024
Estimated Expiration: ⤷ Sign Up
Patent: 0151
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 2006004100
Estimated Expiration: ⤷ Sign Up
Patent: 2006004107
Estimated Expiration: ⤷ Sign Up
Patent: 30206
Estimated Expiration: ⤷ Sign Up
Patent: 19944
Estimated Expiration: ⤷ Sign Up
Patent: 07099781
Estimated Expiration: ⤷ Sign Up
Patent: 12021029
Estimated Expiration: ⤷ Sign Up
Jordan
Patent: 32
Estimated Expiration: ⤷ Sign Up
Malaysia
Patent: 8809
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 06014458
Estimated Expiration: ⤷ Sign Up
Montenegro
Patent: 520
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 0720
Estimated Expiration: ⤷ Sign Up
Norway
Patent: 9101
Estimated Expiration: ⤷ Sign Up
Patent: 065754
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 64361
Estimated Expiration: ⤷ Sign Up
Patent: 72450
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 64361
Estimated Expiration: ⤷ Sign Up
Russian Federation
Patent: 23930
Estimated Expiration: ⤷ Sign Up
Serbia
Patent: 752
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 64361
Estimated Expiration: ⤷ Sign Up
Patent: 72450
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 0609274
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 0939743
Estimated Expiration: ⤷ Sign Up
Patent: 1187218
Estimated Expiration: ⤷ Sign Up
Patent: 070008617
Estimated Expiration: ⤷ Sign Up
Patent: 070028459
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 04697
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 46660
Estimated Expiration: ⤷ Sign Up
Patent: 0606144
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering FYCOMPA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Austria | E547405 | ⤷ Sign Up | |
| Japan | 2007099781 | METHOD FOR PRODUCING CRYSTAL OF 1,2-DIHYDROPYRIDINE COMPOUND | ⤷ Sign Up |
| Israel | 180151 | METHOD FOR PRODUCING 1,2-DIHYDROPYRIDINE-2-ONE COMPOUND | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for FYCOMPA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1764361 | PA2013017,C1764361 | Lithuania | ⤷ Sign Up | PRODUCT NAME: PERAMPANELUM; REGISTRATION NO/DATE: EU/1/12/776/001 - EU/1/12/776/016, 2012 07 23 EU/1/12/776/017 - EU1/12/776/023 20121029 |
| 1300396 | C01300396/01 | Switzerland | ⤷ Sign Up | PRODUCT NAME: PERAMPANEL; REGISTRATION NO/DATE: SWISSMEDIC 62440 14.12.2012 |
| 1300396 | 2012/049 | Ireland | ⤷ Sign Up | PRODUCT NAME: PERAMPANEL, OPTIONALLY A SALT OR HYDRATE THEREOF.; REGISTRATION NO/DATE: EU/1/12/776/001-EU/1/12/776/016 20120723 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


