Last updated: July 28, 2025
Introduction
Fluorouracil (5-FU) is a cornerstone chemotherapeutic agent and pyrimidine analogue widely employed in cancer treatment. Since its approval in the 1950s, fluorouracil has maintained prominence in oncological protocols, particularly for colorectal, breast, gastric, and skin cancers. Recent shifts in market dynamics, driven by emerging biosimilars, novel combination therapies, and advances in targeted treatment, are reshaping fluorouracil’s economic landscape. This analysis delineates the key factors influencing fluorouracil's market trajectory and evaluates future revenue prospects.
Historical Context and Market Overview
As one of the earliest chemotherapies, fluorouracil has established a substantial clinical and commercial footprint. Historically, the drug remained a first-line therapy for various malignancies owing to its efficacy, well-characterized safety profile, and relatively low cost. Its production primarily involves chemical synthesis, with several manufacturers globally, including Sanofi, Teva, and Pfizer, contributing to substantial supply security.
Despite decades of entrenched use, the drug's market experienced gradual stagnation given the advent of targeted therapies and immunotherapies. Nevertheless, fluorouracil persists as a foundational component in treatment regimens, especially in healthcare settings with resource constraints, owing to its affordability and clinical familiarity.
Market Drivers
1. Growing Incidence of Cancer
The global cancer burden is increasing, with the World Health Organization (WHO) reporting an estimated 19.3 million new cancer cases in 2020. Colorectal cancer ranks as the third most diagnosed cancer worldwide, directly correlating with fluorouracil's primary indications. An aging population and lifestyle-related risk factors further amplify incidence rates, bolstering demand for effective chemotherapies like fluorouracil.
2. Established Clinical Protocols and Usage Preference
Fluorouracil's decades-long clinical use confers a high level of physician familiarity. Its inclusion in standard treatment protocols as monotherapy or combination therapy underpins steady demand. The drug’s integration into widely accepted regimens—including FOLFOX (folinic acid, fluorouracil, oxaliplatin)—supports sustained utilization.
3. Cost-Effectiveness and Accessibility
In lower- and middle-income countries, fluorouracil remains a cost-effective option, often favored over newer, expensive agents. Simplified manufacturing processes contribute to low prices, essential in regions with budgetary constraints on healthcare resources.
4. Biosimilars and Competition
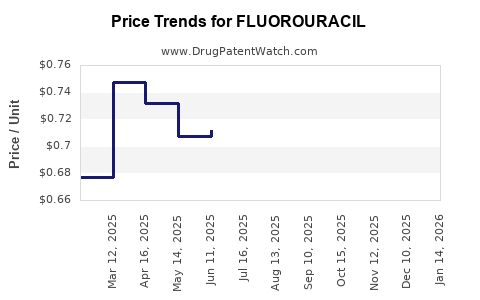
The advent of biosimilar formulations from multiple manufacturers has enhanced market competition, reducing the price point and increasing accessibility. These biosimilars often comply with regulatory standards that ensure therapy equivalence while offering cost benefits.
5. Complementary Role in Combination Therapies
Fluorouracil’s compatibility with monoclonal antibodies (e.g., cetuximab) and targeted agents sustains its demand within multi-drug regimens. This entrenched position bolsters its market resilience amid therapeutic innovations.
Market Challenges
1. Emergence of Targeted and Immunotherapies
Novel agents such as immune checkpoint inhibitors (e.g., pembrolizumab) and targeted therapies significantly challenge fluorouracil's predominance. These therapies often demonstrate superior efficacy and safety profiles, leading to a gradual shift away from traditional chemotherapies, especially in high-income markets.
2. Development of Resistance
Tumor resistance to fluorouracil remains an impediment, prompting investigations into biomarkers predicting response and the development of alternative strategies. Resistance often shortens the clinical utility window for fluorouracil in specific indications.
3. Regulatory and Patent Expirations
While fluorouracil itself is off-patent, regulatory hurdles around biosimilar approvals and market entry logistics may influence pricing strategies and availability.
4. Regional Disparities
Developed regions witness a transition toward targeted therapeutics, whereas low-resource areas maintain reliance on fluorouracil, creating a divergent global market dynamic. Such disparities impact overall market growth and penetration strategies.
Financial Trajectory and Forecasting
Current Revenue Estimates
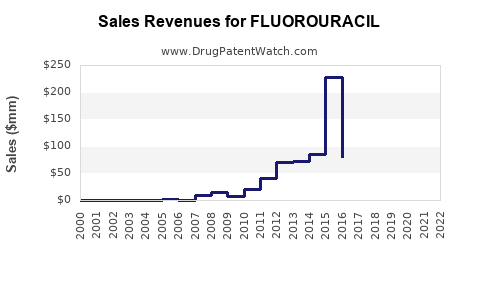
According to recent industry reports, the global fluorouracil market was valued in the vicinity of USD 300-400 million in 2022. Major contributors include North America, Europe, and parts of Asia-Pacific, with revenue driven by increasing cancer incidence and ongoing clinical trials supporting fluorouracil's use.
Future Market Potential
Forecasts suggest a compound annual growth rate (CAGR) of approximately 3-5% over the next five years. Growth is primarily fueled by:
- Continued demand in lower-income countries.
- Increasing adoption of biosimilars, which boost affordability and volume.
- Expansion of fluorouracil's role in combination regimens for new indications.
In high-income markets, however, growth may plateau or decline as newer therapies replace fluorouracil in specific indications, especially where clinical guidelines favor targeted treatments over tradtional chemotherapy.
Factors Influencing Financial Trajectory
The economic outlook hinges on:
- Market penetration of biosimilars: Lower prices will support volume growth but may erode margins.
- Regulatory approvals: Approvals for novel formulations or combination strategies can catalyze sales.
- Clinical adoption: Shifts in standard-of-care protocols directly impact demand.
- Emergence of resistance and efficacy concerns: These may hasten the replacement of fluorouracil in certain indications.
Prognosis of Market Share
While fluorouracil’s overall market volume may experience modest growth, its share in high-income regions is likely to diminish as personalized medicine gains traction. Conversely, in emerging markets, fluorouracil's affordability ensures maintained or marginally enhanced market share.
Strategic Implications for Stakeholders
Manufacturers should prioritize biosimilar development to capture cost-sensitive markets and invest in formulations suitable for combination therapies. Healthcare providers must balance efficacy, safety, and cost to optimize treatment landscapes, potentially integrating fluorouracil in resource-limited settings. Policymakers can influence market dynamics through regulatory pathways that encourage biosimilar entry and support equitable access to cancer therapies.
Conclusion
The market dynamics and financial outlook for fluorouracil are characterized by a combination of enduring clinical utility and evolving therapeutic competition. While traditional chemotherapy remains relevant globally, especially in resource-constrained settings, high-income markets are gradually transitioning toward targeted therapies. Nonetheless, fluorouracil's future remains stable, supported by biosimilar proliferation, ongoing clinical roles, and its cost-effective profile. Stakeholders must continuously monitor evolving treatment protocols, regulatory environments, and technological innovations to strategically navigate this complex landscape.
Key Takeaways
- Fluorouracil maintains a substantial market due to its long-standing efficacy, widespread clinical familiarity, and cost advantages.
- Growth prospects are moderate, with forecasts indicating a CAGR of 3-5% over five years, primarily driven by emerging markets and biosimilar competition.
- Dominant challenges include competition from targeted therapies, resistance issues, and evolving clinical guidelines favoring newer agents.
- Strategic focus on biosimilar expansion, combination therapy integration, and geographic market penetration will shape fluorouracil’s financial trajectory.
- Stakeholders should tailor strategies aligned with regional healthcare policies, market demands, and technological innovations to optimize value and market share.
FAQs
1. How does the rise of biosimilars impact fluorouracil's market?
Biosimilars reduce manufacturing costs and price points, enhancing accessibility—especially in cost-sensitive regions—potentially increasing volume but pressuring traditional profit margins for original manufacturers.
2. Are there new formulations of fluorouracil emerging?
Yes. Innovations include topical formulations for skin cancers and microencapsulated versions aimed at improved stability and delivery, which can expand clinical applications and improve patient compliance.
3. How does fluorouracil compare to newer targeted therapies?
While fluorouracil remains effective and economical, targeted therapies often demonstrate superior efficacy and fewer side effects, leading to their preferential use in developed markets where cost is less restrictive.
4. What role does fluorouracil play in combination regimens?
It continues to be a fundamental component in multi-drug regimens like FOLFOX and FOLFIRI, especially for colorectal cancers, reinforcing its market presence despite newer alternatives.
5. Will fluorouracil become obsolete?
Not imminently. Its low cost, established efficacy, and integration into treatment protocols suggest it will remain relevant, particularly in resource-limited settings or as part of combination therapies.
References
[1] World Health Organization. Cancer Fact Sheet. 2020.
[2] MarketWatch. Fluorouracil Market Size, Share & Trends Analysis. 2023.
[3] Oncology Protocols and Guidelines. NCCN. 2022.
[4] Global Data. Biosimilars in Oncology: Market Trends. 2022.