Last updated: July 27, 2025
Introduction
Felodipine, a calcium channel blocker, is commonly indicated for hypertension and angina. Since its initial approval in the late 1980s, felodipine has maintained its relevance in cardiovascular therapeutics owing to its efficacy and favorable safety profile. As a generic drug predominantly marketed by several pharmaceutical giants, understanding its market dynamics and financial trajectory offers insights into broader cardiovascular medication trends and generic drug markets.
Pharmacological Profile and Therapeutic Positioning
Felodipine’s mechanism involves selective inhibition of L-type calcium channels in vascular smooth muscle, resulting in vasodilation and reduced blood pressure. It is classified within dihydropyridine calcium channel blockers, with a longer half-life supporting once-daily dosing. Its clinical utility extends to managing hypertension, often in combination therapies, with a robust safety profile characterized by minimal cardiac conduction effects.
The drug’s patent expiry in the early 2000s catalyzed a shift towards generic manufacturing, significantly impacting its market size and competitive landscape.
Market Overview
Global and Regional Market Size
The global antihypertensive drugs market is projected to reach approximately USD 34 billion by 2028, growing at a CAGR of 3.9%[1]. Felodipine, as a well-established drug, occupies a notable share within the calcium channel blocker segment, which itself constitutes roughly 23% of the antihypertensive market[2].
Regionally, North America and Europe dominate due to high prevalence of hypertension, extensive healthcare infrastructure, and widespread generic drug adoption. Asia-Pacific markets are emerging as significant players owing to increasing hypertension prevalence, rising healthcare investments, and expanding pharmaceutical manufacturing capabilities.
Market Drivers
-
Chronic Disease Burden: The global hypertension burden continues to increase, with the World Health Organization estimating over 1.2 billion affected individuals worldwide. This sustains demand for long-term antihypertensive therapies like felodipine.
-
Cost-Effectiveness and Generics: Post-patent expiration, felodipine’s generic versions have markedly reduced treatment costs, encouraging broader adoption across healthcare systems with constrained budgets.
-
Combination Therapies: With hypertension often requiring multi-drug regimens, felodipine’s compatibility with other antihypertensives amplifies its market potential.
-
Regulatory Approvals: Expanding indications or formulations can stimulate market growth. For example, formulations with extended-release technology improve patient compliance.
Market Challenges
-
Intense Competition: The presence of multiple branded and generic calcium channel blockers, such as amlodipine, nifedipine, and nifedipine, creates a highly competitive environment.
-
Price Wars: The entry of numerous low-cost generics compresses margins and limits revenue potential for producers.
-
Patent Landscape: Although felodipine’s main patent has expired, secondary patents or formulation-specific exclusivities can temporarily shield certain products.
-
Pricing and Reimbursement Dynamics: Payer pressure in developed markets influences pricing strategies, often favoring cheaper generics.
Financial Trajectory and Revenue Trends
Historical Revenue Patterns
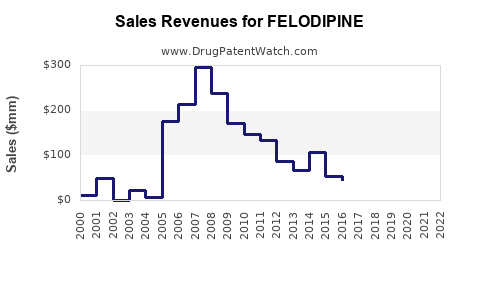
Following patent expiration in the early 2000s, felodipine experienced a sharp decline in branded sales, with market share shifting towards generics. Major pharmaceutical firms such as AstraZeneca (original developer) transitioned their focus to newer molecules but maintained a presence via generic lines.
In emerging markets, local manufacturers have seized opportunities, with revenues stabilized by high-volume, low-margin sales. For instance, India and China account for a sizable share of felodipine manufacturing, contributing significantly to regional revenues.
Current Market Performance
-
Revenue Stability: Due to widespread generic availability, individual product revenues are often modest but remain stable due to sustained demand. The global generic calcium channel blocker market size was valued at approximately USD 8 billion in 2021, with felodipine representing roughly 15-20%[3].
-
Profit Margins: Margins have compressed, with companies shifting focus to high-margin innovator drugs or novel therapies. However, cost-efficient manufacturing and high-volume sales sustain profitability for generic producers.
-
Impact of Patent Challenges: Legal disputes and patent litigations in certain jurisdictions occasionally influence revenues, but overall impact remains limited given the drug’s generic status.
Future Revenue Drivers
-
Formulation Innovations: Extended-release formulations and combination pills can generate incremental revenue streams.
-
Market Expansion: Entry into developing markets with rising hypertension prevalence can bolster revenues.
-
Strategic Partnerships: Collaborations with local manufacturers can facilitate distribution expansion and market penetration.
Regulatory and Patent Landscape
Felodipine’s original patent protection expired around 2001, leading to a proliferation of generic versions globally. Patents related to specific formulations or delivery methods can extend exclusivity periods marginally. Regulatory approval pathways for generics—via ANDA filings in the US, for example—have facilitated rapid market entry post-patent expiry.
This landscape influences market competition and pricing strategies, often resulting in aggressive price reductions to maintain share.
Competitive Landscape
Besides felodipine, the calcium channel blocker class includes drugs like amlodipine, nifedipine, and nimodipine. Amlodipine dominates due to its favorable pharmacokinetics and once-daily dosing. Nonetheless, felodipine retains niche advantages for certain patient populations due to its minimal reflex tachycardia effects.
Generic competition has fragmented the market, with major players including Teva, Mylan, and local manufacturers offering low-cost formulations.
Impact of Emerging Technologies and Trends
-
Personalized Medicine: Pharmacogenomic insights could influence prescribing patterns, potentially affecting demand.
-
Digital Therapeutics Integration: Remote monitoring and adherence programs could increase long-term therapy continuity, benefiting drugs like felodipine.
-
Public Health Initiatives: International efforts to control hypertension may lead to increased prescription rates, especially in low- and middle-income countries.
Forecast and Strategic Outlook
-
Short-Term (1-3 Years): Revenue remains steady, driven by generic sales in mature markets. Price pressures are expected to persist, but formulation innovations and market expansion efforts may mitigate declines.
-
Medium to Long-Term (3-10 Years): Growth could stem from new combination therapies, improved formulations, and expanding market access in emerging economies. Patent litigations or new formulation patents may temporarily influence market shares.
-
Risks: Price erosion, regulatory reforms, and competition from novel antihypertensive agents pose ongoing challenges.
Key Takeaways
-
Market Size Stability: Post-patent expiration, felodipine maintains a stable market presence driven by its efficacy, safety profile, and affordability.
-
Intense Competition: Dominated by generic manufacturers, with significant price competition limiting margins.
-
Emerging Market Opportunities: Rising hypertension prevalence and healthcare infrastructure improvements create avenues for growth.
-
Formulation and Combination Opportunities: Innovations can offer competitive advantages and new revenue streams.
-
Regulatory and Patent Landscape: Continual legal and regulatory vigilance remains essential to sustain market position.
FAQs
1. How has patent expiration impacted felodipine’s market share?
Patent expiration in the early 2000s led to a surge in generic manufacturing, substantially reducing branded sales but expanding overall access. Market share shifted toward low-cost generics, resulting in a more fragmented but stable market.
2. What are the key competitive advantages of felodipine over other calcium channel blockers?
Felodipine offers selective vasodilation with minimal reflex tachycardia, favorable pharmacokinetics supporting once-daily dosing, and a safety profile suitable for long-term management, especially in certain patient subsets.
3. How do emerging markets influence felodipine’s financial trajectory?
Emerging markets present increased demand owing to rising hypertension prevalence and cost-sensitive healthcare systems. Local manufacturing and regulatory approvals are facilitating market expansion, sustaining revenues.
4. What innovations are augmenting felodipine’s market prospects?
Extended-release formulations, fixed-dose combination therapies, and targeted marketing strategies are enhancing its therapeutic profile, patient compliance, and sales volume.
5. What challenges face manufacturers of felodipine in maintaining profitability?
Intense price competition, declining margins due to generics, regulatory hurdles, and competition from newer agents pose ongoing profitability challenges. Strategic differentiation through formulation technology and market expansion is necessary.
References
[1] Grand View Research. "Antihypertensive Drugs Market Size, Share & Trends Analysis Report." 2022.
[2] IQVIA. "Global Cardiovascular Therapeutics Market Report." 2022.
[3] MarketsandMarkets. "Calcium Channel Blockers Market by Type, Application, and Region." 2021.