Last updated: July 27, 2025
Introduction
Erythromycin, a macrolide antibiotic discovered in the 1950s, remains a pivotal drug in infectious disease management. Despite the advent of newer antibiotics, erythromycin maintains relevance owing to its efficacy, safety profile, and extensive historical data. This report examines the current market dynamics, competitive landscape, regulatory factors, and forecasted financial trajectory of erythromycin within the global pharmaceutical industry.
Historical Context and Therapeutic Role
Erythromycin’s significance stemmed from its broad-spectrum antibacterial activity, especially against atypical pathogens such as Mycoplasma, Chlamydia, and Legionella. Its introduction revolutionized outpatient infections management, providing a safe alternative for penicillin-allergic patients (1). Over the decades, erythromycin formulations—originally in oral and injectable forms—have faced competition from newer macrolides like azithromycin and clarithromycin, which offer improved dosing schedules and fewer side effects. Nevertheless, erythromycin persists as a first-line option in certain clinical scenarios and remains widely prescribed in developing countries with limited access to newer agents.
Current Market Dynamics
Global Market Size and Growth
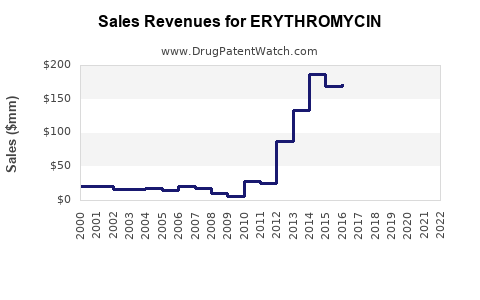
The erythromycin market experienced fluctuations over the past decade, largely influenced by antibiotic stewardship initiatives, generic drug competition, and evolving clinical guidelines. According to recent industry reports, the global erythromycin market was valued at approximately $350 million in 2022, with a compound annual growth rate (CAGR) estimated at 2-3% through 2028 (2). The steady growth primarily reflects demand in emerging markets where antibiotics are integral to primary healthcare infrastructure.
Regional Market Trends
-
North America & Europe: Market saturation and mature pharmaceutical landscapes have led to aggressive generic competition. Erythromycin prescriptions are declining proportionally due to the preference for newer antibiotics with better tolerability and dosing convenience (3). Regulatory bodies emphasize antimicrobial stewardship, further limiting erythromycin’s regional growth prospects.
-
Asia-Pacific & Latin America: These regions represent the fastest-growing segments, driven by expanding healthcare access, increased infectious disease burden, and affordability of generic formulations. Countries like India, China, and Brazil are major markets, with local manufacturers producing erythromycin at lower costs to meet rising demand (4).
Competitive Landscape
The market is predominantly populated by generic manufacturers who benefit from patent expirations dating back decades—most notably the original patent expired in the early 2000s. Big pharmaceutical companies, including Teva, Mylan, and Sun Pharmaceutical, hold significant share via globally recognized manufacturing capabilities. The market entry barriers are relatively low, fostering a highly competitive environment that exerts downward pressure on prices.
Drug Formulations and Patent Landscape
Erythromycin remains available in various formulations: tablets, suspensions, topical ointments, and injectables. While no further patent protections exist, formulations with modified release or combination therapies are areas of ongoing innovation. Biotech firms are exploring erythromycin derivatives to improve pharmacokinetic profiles, though none have reached commercialization significant enough to alter current dynamics substantially.
Regulatory and Prescriptive Trends
Regulatory agencies, including the FDA and EMA, advocate judicious antibiotic use to combat antimicrobial resistance (AMR). This stance influences prescribing practices, often favoring newer agents with more specific activity spectra or better safety profiles. Consequently, erythromycin’s utilization is gradually declining in hospitals and outpatient settings within developed countries, although its role in community settings persists.
Financial Trajectory and Future Outlook
Revenue Projections
The erythromycin market is projected to grow modestly at a CAGR of 2-3% over the next five years. The main drivers include:
- Continued demand in emerging markets
- Stable generic supply chains
- Expansion of erythromycin use in veterinary medicine (which accounts for an estimated 5-10% of total erythromycin sales, depending on regional regulations) (5)
However, growth is constrained by:
- Declining prescriptions in the United States and Europe due to stewardship programs
- Rising concerns over antibiotic resistance, which may restrict indications (6)
Pricing Trends
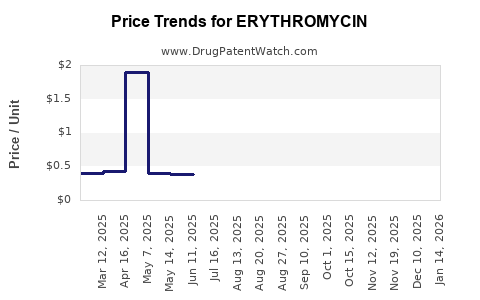
Market prices are under persistent pressure due to generic competition. The average wholesale price (AWP) for erythromycin tablets has declined by approximately 30% over the past decade, with further declines anticipated. Margins remain narrow, emphasizing reliance on high-volume sales strategies especially in generics manufacturing.
Emerging Opportunities
- Development of Ester Derivatives and New Formulations: These aim to improve bioavailability, reduce adverse effects, and extend patent life.
- Use in Combination Therapies: Combining erythromycin with other antimicrobial agents to combat resistant strains could rejuvenate market interest.
- Veterinary Applications: The persistent use in livestock and pet medicine presents an alternative revenue stream, especially in regions with favorable regulatory environments.
Challenges Impacting Financial Trajectory
- Antimicrobial Resistance: Erythromycin resistance rates are rising globally, especially among Streptococcus pneumoniae and Staphylococcus aureus, limiting clinical efficacy and thus market size (7).
- Early Adoption of Targeted Therapy: Precision medicine approaches favor narrow-spectrum drugs, reducing erythromycin’s utility.
- Regulatory Pushback: Stricter guidelines on antibiotic use decrease prescription volumes.
Strategic Considerations for Stakeholders
- Manufacturers: Focus on formulations with improved safety profiles or novel delivery mechanisms.
- Investors: Assess regional market drivers, especially in countries with rising healthcare access and broad antibiotic use.
- Healthcare Policy Makers: Implement stewardship programs that balance infection control with appropriate erythromycin utilization to optimize financial returns without fostering resistance.
Conclusion
Erythromycin’s market landscape is characterized by stable but moderate growth driven by emerging markets, with declining prominence in developed countries. Generic manufacturing and regional healthcare trends dictate its financial trajectory, with narrow margins and resistance concerns shaping future potential. Stakeholders should prioritize innovation within formulation sciences and strategic market expansion to sustain profitability in this mature but enduring antibiotic segment.
Key Takeaways
- The erythromycin market is projected to grow at a modest CAGR of 2-3% through 2028, primarily fueled by demand in emerging economies.
- Brand and generic competition significantly suppress prices, constraining profit margins.
- Rising antimicrobial resistance and stricter prescribing guidelines are reducing erythromycin’s usage in developed markets.
- Opportunities exist in new formulations, combination therapies, and veterinary applications, which can help prolong commercial viability.
- Stakeholders must navigate regulatory environments carefully, balancing antimicrobial stewardship with market sustainability.
FAQs
1. What factors are driving erythromycin's renewed relevance in certain markets?
Emerging economies with increasing healthcare access and less stringent antibiotic regulations sustain consistent demand, especially where affordable generic options meet local needs.
2. How does antimicrobial resistance affect erythromycin's market prospects?
Rising resistance diminishes erythromycin’s clinical effectiveness, leading to reduced prescriptions and limiting market growth, especially in respiratory and skin infections.
3. Are there any recent innovations in erythromycin formulations?
While no groundbreaking formulations have reached widespread adoption, research into ester derivatives and sustained-release formulations aims to boost efficacy and minimize side effects.
4. How is the patent landscape influencing erythromycin's market?
The original patents expired decades ago, resulting in intense generic competition that sustains low pricing but limits high-margin opportunities.
5. What strategic moves can pharmaceutical companies consider to maximize erythromycin’s value?
Innovation in formulation, exploring combination therapies, and expanding veterinary applications can extend erythromycin’s lifecycle amid market challenges.
References
- [1] Goodman & Gilman's The Pharmacological Basis of Therapeutics, 13th Edition.
- [2] Industry Reports: Global Antibiotics Market Analysis, 2023.
- [3] CDC Antibiotic Prescribing Data, 2022.
- [4] MarketsandMarkets: Asia-Pacific Antibiotics Market, 2023.
- [5] Veterinary Antimicrobials Market Report, 2022.
- [6] World Health Organization: Antimicrobial Resistance Surveillance, 2021.
- [7] Clinical Infectious Diseases Journal: Resistance Trends in Macrolide-Resistant Bacteria, 2022.