ELESTRIN Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Elestrin, and what generic alternatives are available?
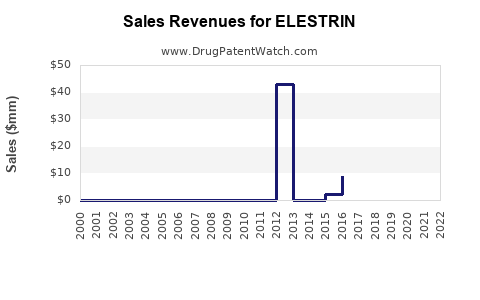
Elestrin is a drug marketed by Mylan Speciality Lp and is included in one NDA.
The generic ingredient in ELESTRIN is estradiol. There are seventy-five drug master file entries for this compound. Forty-six suppliers are listed for this compound. Additional details are available on the estradiol profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Elestrin
A generic version of ELESTRIN was approved as estradiol by BARR LABS INC on October 22nd, 1997.
Summary for ELESTRIN
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 109 |
| Clinical Trials: | 1 |
| Patent Applications: | 4,193 |
| Formulation / Manufacturing: | see details |
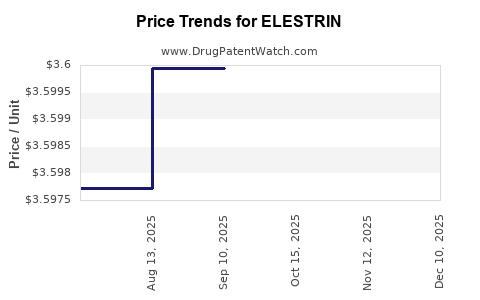
| Drug Prices: | Drug price information for ELESTRIN |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ELESTRIN |
| What excipients (inactive ingredients) are in ELESTRIN? | ELESTRIN excipients list |
| DailyMed Link: | ELESTRIN at DailyMed |
Recent Clinical Trials for ELESTRIN
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Missouri-Columbia | N/A |
Pharmacology for ELESTRIN
| Drug Class | Estrogen |
| Mechanism of Action | Estrogen Receptor Agonists |
Anatomical Therapeutic Chemical (ATC) Classes for ELESTRIN
US Patents and Regulatory Information for ELESTRIN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mylan Speciality Lp | ELESTRIN | estradiol | GEL, METERED;TRANSDERMAL | 021813-001 | Dec 15, 2006 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ELESTRIN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Mylan Speciality Lp | ELESTRIN | estradiol | GEL, METERED;TRANSDERMAL | 021813-001 | Dec 15, 2006 | ⤷ Sign Up | ⤷ Sign Up |
| Mylan Speciality Lp | ELESTRIN | estradiol | GEL, METERED;TRANSDERMAL | 021813-001 | Dec 15, 2006 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for ELESTRIN
See the table below for patents covering ELESTRIN around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Mexico | PA06003316 | FORMULACION FARMACEUTICA TRANSDERMICA PARA MINIMIZAR LOS RESIDUOS EN LA PIEL. (TRANSDERMAL PHARMACEUTICAL FORMULATION FOR MINIMIZING SKIN RESIDUES.) | ⤷ Sign Up |
| South Korea | 20030031568 | ⤷ Sign Up | |
| European Patent Office | 1325752 | ⤷ Sign Up | |
| Australia | 2004220498 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ELESTRIN
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1453521 | 300814 | Netherlands | ⤷ Sign Up | PRODUCT NAME: LEVONORGESTREL EN ETHINYLESTRADIOL; NATIONAL REGISTRATION NO/DATE: RVG 117453 20151211; FIRST REGISTRATION: SK 17/0017/15-S 20150211 |
| 2782584 | 122021000080 | Germany | ⤷ Sign Up | PRODUCT NAME: ZUSAMMENSETZUNG, DIE SOWOHL ESTRADIOL (17SS-ESTRADIOL), GEGEBENENFALLS IN FORM EINES PHARMAZEUTISCH ANNEHMBAREN SALZES, HYDRATS ODER SOLVATS DAVON (EINSCHLIESSLICH IN FORM EINES HEMIHYDRATS), ALS AUCH PROGESTERON ENTHAELT; NAT. REGISTRATION NO/DATE: 2205034.00.00 20210924; FIRST REGISTRATION: BELGIEN BE582231 20210406 |
| 0398460 | C300221 | Netherlands | ⤷ Sign Up | PRODUCT NAME: DROSPIRENON EN ETHINYLESTRADIOL; REGISTRATION NO/DATE: RVG 23827 20000307 |
| 2782584 | C202130068 | Spain | ⤷ Sign Up | PRODUCT NAME: COMPOSICION QUE CONTIENE ESTRADIOL (17BETA-ESTRADIOL), INCLUYENDO EN FORMA DE HEMIHIDRATO, Y PROGESTERONA; NATIONAL AUTHORISATION NUMBER: 85988-NL/H/4994/001/DC; DATE OF AUTHORISATION: 20210528; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): BE582231; DATE OF FIRST AUTHORISATION IN EEA: 20210406 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


