DAPTOMYCIN Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Daptomycin, and when can generic versions of Daptomycin launch?
Daptomycin is a drug marketed by Accord Hlthcare, Aspiro, Be Pharms, Biocon Pharma, Dr Reddys, Eugia Pharma, Fresenius Kabi Usa, Hainan Poly Pharm, Hangzhou Zhongmei, Hengrui Pharma, Hikma, Hisun Pharm Hangzhou, Hospira, Maia Pharms Inc, Meitheal, Mylan, Mylan Labs Ltd, Qilu Pharm Hainan, Sagent Pharms Inc, Teva Pharms Usa, Xellia Pharms Aps, and Baxter Hlthcare Corp. and is included in thirty-one NDAs. There are four patents protecting this drug.
This drug has forty patent family members in twenty-four countries.
The generic ingredient in DAPTOMYCIN is daptomycin. There are ten drug master file entries for this compound. Twenty-eight suppliers are listed for this compound. Additional details are available on the daptomycin profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Daptomycin
A generic version of DAPTOMYCIN was approved as daptomycin by TEVA PHARMS USA on March 25th, 2016.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for DAPTOMYCIN?
- What are the global sales for DAPTOMYCIN?
- What is Average Wholesale Price for DAPTOMYCIN?
Summary for DAPTOMYCIN
| International Patents: | 40 |
| US Patents: | 4 |
| Applicants: | 22 |
| NDAs: | 31 |
| Finished Product Suppliers / Packagers: | 27 |
| Raw Ingredient (Bulk) Api Vendors: | 48 |
| Clinical Trials: | 94 |
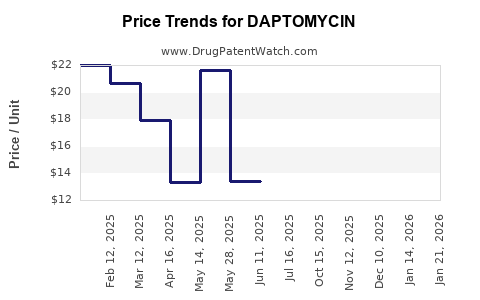
| Drug Prices: | Drug price information for DAPTOMYCIN |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for DAPTOMYCIN |
| What excipients (inactive ingredients) are in DAPTOMYCIN? | DAPTOMYCIN excipients list |
| DailyMed Link: | DAPTOMYCIN at DailyMed |
Recent Clinical Trials for DAPTOMYCIN
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University Clinical Hospital na V.V.Vinogradov (branch of RUDN university na Patrice Lumumba) | PHASE3 |
| University of Melbourne | PHASE4 |
| The Peter Doherty Institute for Infection and Immunity | PHASE4 |
Pharmacology for DAPTOMYCIN
| Drug Class | Lipopeptide Antibacterial |
Medical Subject Heading (MeSH) Categories for DAPTOMYCIN
Anatomical Therapeutic Chemical (ATC) Classes for DAPTOMYCIN
Paragraph IV (Patent) Challenges for DAPTOMYCIN
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| CUBICIN RF | For Injection | daptomycin | 500 mg/vial | 021572 | 1 | 2008-11-19 |
US Patents and Regulatory Information for DAPTOMYCIN
DAPTOMYCIN is protected by four US patents.
EU/EMA Drug Approvals for DAPTOMYCIN
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Pfizer Europe MA EEIG | Daptomycin Hospira | daptomycin | EMEA/H/C/004310Daptomycin is indicated for the treatment of the following infections.Adult and paediatric (1 to 17 years of age) patients with complicated skin and soft-tissue infections (cSSTI).Adult patients with right-sided infective endocarditis (RIE) due to Staphylococcus aureus. It isrecommended that the decision to use daptomycin should take into account the antibacterial susceptibility of the organism and should be based on expert advice.Adult and paediatric (1 to 17 years of age) patients with Staphylococcus aureus bacteraemia (SAB).In adults, use in bacteraemia should be associated with RIE or with cSSTI, while in paediatric patients, use in bacteraemia should be associated with cSSTI.Daptomycin is active against Gram positive bacteria only. In mixed infections where Gram negative and/or certain types of anaerobic bacteria are suspected, daptomycin should be co-administered with appropriate antibacterial agent(s).Consideration should be given to official guidance on the appropriate use of antibacterial agents. | Authorised | yes | no | no | 2017-03-22 | |
| Merck Sharp & Dohme B.V. | Cubicin | daptomycin | EMEA/H/C/000637Cubicin is indicated for the treatment of the following infections.Adult and paediatric (1 to 17 years of age) patients with complicated skin and soft-tissue infections (cSSTI).Adult patients with right-sided infective endocarditis (RIE) due to Staphylococcus aureus.It is recommended that the decision to use daptomycin should take into account the antibacterial susceptibility of the organism and should be based on expert advice.Adult and paediatric (1 to 17 years of age) patients with Staphylococcus aureus bacteraemia (SAB). In adults, use in bacteraemia should be associated with RIE or with cSSTI, while in paediatric patients, use in bacteraemia should be associated with cSSTI.Daptomycin is active against Gram positive bacteria only. In mixed infections where Gram negative and/or certain types of anaerobic bacteria are suspected, Cubicin should be co-administered with appropriate antibacterial agent(s). Consideration should be given to official guidance on the appropriate use of antibacterial agents. | Authorised | no | no | no | 2006-01-19 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for DAPTOMYCIN
See the table below for patents covering DAPTOMYCIN around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 3675815 | ⤷ Get Started Free | |
| China | 118766886 | ⤷ Get Started Free | |
| South Africa | 201502310 | DAPTOMYCIN FORMULATIONS AND USES THEREOF | ⤷ Get Started Free |
| China | 111093625 | ⤷ Get Started Free | |
| Denmark | 2895187 | ⤷ Get Started Free | |
| Canada | 3070660 | ⤷ Get Started Free | |
| Hungary | E13837694 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for DAPTOMYCIN
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1115417 | SZ 22/2006 | Austria | ⤷ Get Started Free | PRODUCT NAME: DAPTOMYCIN |
| 1115417 | CA 2006 00018 | Denmark | ⤷ Get Started Free | PRODUCT NAME: DAPTOMYCIN |
| 1115417 | 22/2006 | Austria | ⤷ Get Started Free | PRODUCT NAME: DAPTOMYCIN; REGISTRATION NO/DATE: EU/1/05/328/001 UND 002 20060119 |
| 1115417 | SPC/GB06/024 | United Kingdom | ⤷ Get Started Free | SUPPLEMENTARY PROTECTION CERTIFICATE NO SPC/GB06/024 GRANTED TO CUBIST PHARMACEUTICALS, INC IN RESPECT OF THE PRODUCT DAPTOMYCIN, THE GRANT OF WHICH WAS ADVERTISED IN JOURNAL NO 6162 DATED 27 JUNE 2007 HAS HAD ITS MAXIMUM PERIOD OF DURATION CORRECTED, SUBJECT TO THE PAYMENT OF THE PRESCRIBED FEES IT WILL EXPIRE ON 22 JANUARY 2021. |
| 1115417 | 06C0022 | France | ⤷ Get Started Free | PRODUCT NAME: DAPTOMYCINE; REGISTRATION NO/DATE: EU/1/05/328/001-002 20060119 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for Daptomycin
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
