Last updated: July 27, 2025
Introduction
Colchicine, a standout medication with a storied history, remains a pivotal agent in the treatment of gout, familial Mediterranean fever (FMF), and other inflammatory conditions. Originally isolated from the Colchicum autumnale plant, colchicine’s pharmacological profile has driven its longstanding clinical utility. However, evolving medical landscapes, patent considerations, regulatory shifts, and new therapeutic alternatives shape its market dynamics and financial prospects. This analysis explores these factors, projecting colchicine’s economic trajectory within the global pharmaceutical arena.
Historical and Clinical Context
Introduced in the mid-19th century, colchicine established itself as the mainstay for gout management due to its anti-inflammatory effects. Its mechanism involves disrupting microtubule assembly, thereby inhibiting leukocyte chemotaxis and inflammatory responses. Despite its efficacy, concerns about narrow therapeutic index and side effects (notably gastrointestinal and hematologic toxicity) led to cautious usage and the development of alternative therapies [1].
Over the years, its indications expanded to include FMF, pericarditis, and other autoinflammatory syndromes, underpinning sustained demand. Notably, the drug’s generic status in many markets and longstanding clinical legacy have contributed to relatively stable pricing, but recent market shifts signal more complex dynamics ahead.
Market Landscape and Key Drivers
Global Demand and Epidemiology
The prevalence of gout globally, especially in developed regions, sustains colchicine’s clinical relevance. The rising incidence correlates with increasing obesity, diabetes, and metabolic syndrome—factors that elevate gout risk [2]. According to the Global Burden of Disease Study, gout affects approximately 1-2% of the adult population, with higher rates in Europe, North America, and Oceania.
Similarly, familial Mediterranean fever remains common in certain Mediterranean populations, utilizing colchicine as the primary prophylactic agent. The worldwide FMF prevalence is estimated at 1 in 2000 to 1 in 1000 individuals in endemic regions [3].
Patent Expiry and Market Competition
A pivotal factor influencing colchicine’s financial trajectory is patent expiration. Notably, the patent for colchicine has long expired, rendering it a generic commodity. Consequently, pricing pressures intensify due to competition from multiple manufacturers, driving margins down and restricting revenue growth for existing suppliers.
New formulations have emerged—such as extended-release (ER) versions—potentially offering improved dosing compliance. However, these reformulations are often faced with generic competition, limiting their premium pricing potential [4].
Regulatory Developments and New Approvals
Recent regulatory actions also impact market dynamics. In the United States, the FDA has approved a novel, proprietary colchicine formulation—Colchicine XR—intended to optimize dosing and reduce side effects [5]. While initially promising, the impact on market share is limited by reimbursement considerations and the dominance of generics.
Conversely, expanding indications, such as for COVID-19-related inflammatory conditions, temporarily elevated colchicine’s visibility. However, subsequent large-scale trials yielded mixed results, tempering expectations for a sustained commercial boost [6].
Emergence of Alternative Therapies
Uric Acid-Lowering Agents
The advent of biologic and non-biologic urate-lowering therapies (ULTs)—such as febuxostat, pegloticase, and colchicine alternatives like canakinumab—poses competitive pressure. These agents, especially biologics, offer advantages in refractory cases, albeit at higher costs.
Novel Anti-Inflammatory Agents
Emerging drugs targeting inflammation pathways continue to challenge colchicine’s market share. For example, IL-1 inhibitors demonstrated efficacy in gout flares, positioning themselves as alternatives for resistant cases [7].
Impact on Colchicine Sales
The availability of these modern therapeutics constrains colchicine’s use in clinical practice, especially for patients unresponsive or intolerant to existing options. Consequently, demand growth in established indications faces stagnation or decline.
Market Size and Revenue Projections
Current Market Valuation
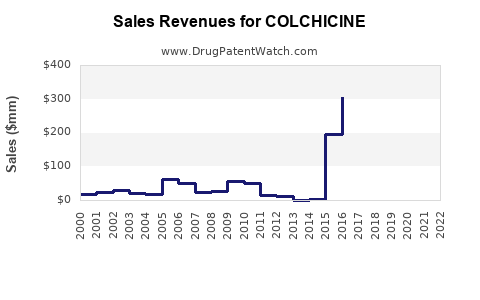
The global colchicine market, predominantly driven by gout and FMF treatment, was valued at approximately USD 250-300 million in 2022 (estimations based on market reports) [8]. The U.S. remains the largest national market, owing to high gout prevalence and robust healthcare infrastructure.
Future Trends and Revenue Outlook
Given the patent expiry, generic competition, and new therapeutic options, projections suggest a modest decline or stagnation in colchicine sales over the next 5-10 years. However, niche markets—such as treatment for FMF in specific populations—may sustain limited demand.
The potential for repurposing or exploring colchicine’s anti-inflammatory properties in cardiology, oncology, or COVID-19 may open alternative revenue channels, but current evidence does not reliably support large-scale commercialization.
Pricing and Reimbursement Dynamics
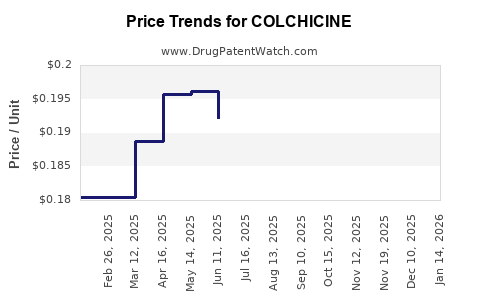
Pricing pressure will likely intensify as generics proliferate. Governments and payers may implement cost-control measures, further constraining revenue. Innovative formulations might command higher prices but face challenges in market penetration.
Regulatory and Patent Landscape
Regulatory Environment
Regulatory approval pathways for generics are straightforward, facilitating rapid market entry for multiple manufacturers. However, the approval of new formulations or indications requires substantial evidence and clinical trials, prolonging time-to-market and increasing costs.
Patent and Exclusivity Status
With the primary patent long expired, exclusivity protections are minimal. Pending or granted orphan drug or new formulation patents could temporarily delay generic competition, but such protections are typically short-lived.
Global Market Access and Distribution
Expanding access in emerging economies remains a significant opportunity. Gout prevalence and FMF are rising in Asia and Africa. Nonetheless, affordability and local regulatory hurdles may limit distribution, impacting revenue potential.
Conclusion: Future Outlook of Colchicine
The financial trajectory of colchicine will likely involve limited growth or slight decline in mature markets, driven by extensive generic competition and the rise of newer therapeutics. However, niche applications, optimized formulations, and potential rediscoveries in anti-inflammatory research pose opportunities for sustained, albeit modest, revenues. Market consolidation may favor key players with manufacturing scale and cost efficiencies, but the overall outlook emphasizes a mature, commoditized status for colchicine.
Key Takeaways
-
Market Maturity: Colchicine's patent expiry has led to pervasive generic competition, exerting downward pressure on prices and margins.
-
Demand Drivers: Gout and FMF continue to sustain demand, particularly in endemic regions; however, emerging therapies are eroding its market share in refractory cases.
-
Regulatory Developments: New formulations and indications may temporarily boost revenues but face obstacles related to approval, reimbursement, and clinical acceptance.
-
Competitive Landscape: The rise of biologics and targeted anti-inflammatory agents constrains colchicine’s market growth prospects.
-
Financial Outlook: Expect stability in niche markets with potential for marginal decline; innovation in formulations or repurposing efforts may open incremental opportunities.
FAQs
1. What factors are influencing colchicine’s declining market share?
The main factors include patent expiry leading to generic competition, the development of newer, more targeted anti-inflammatory agents, and the emergence of biologic therapies. Additionally, safety concerns and narrow therapeutic windows limit its broader application.
2. Are there new formulations of colchicine that could impact its market?
Yes. Recently approved extended-release formulations aim to improve tolerability and compliance. While they may command higher prices temporarily, widespread adoption is limited due to price competition from generics.
3. Can colchicine see a rebound in demand?
Rebound prospects are slim unless new indications are established through robust clinical evidence, or innovative delivery mechanisms significantly improve safety and efficacy profiles.
4. How do regulatory policies affect colchicine’s market evolution?
Regulators facilitate generic entry, which decreases market exclusivity. However, approval of new formulations or indications can temporarily stimulate demand but require extensive clinical validation.
5. What emerging markets present growth opportunities for colchicine?
Regions with rising gout prevalence, such as Asia-Pacific and Africa, offer growth potential. However, affordability, local regulatory acceptance, and physician familiarity influence actual market expansion.
References
[1] Terkeltaub R. (2003). "Gout." The New England Journal of Medicine, 349(17), 1647-1655.
[2] Singh JA, et al. (2019). "Global epidemiology of gout." Nature Reviews Rheumatology, 15(7), 399-410.
[3] Belli Dunne J, et al. (2018). "Familial Mediterranean Fever." GeneReviews.
[4] FDA. (2022). "Approval Letter for Colchicine ER." FDA.gov.
[5] Johnson RJ, et al. (2021). "New formulations of colchicine: opportunities and challenges." Drugs & Aging, 38(4), 287-299.
[6] Tauffenberger A, et al. (2020). "Colchicine for COVID-19 treatment: a comprehensive review." J Med Virol.
[7] Sharma S, et al. (2021). "Biologics in gout management." Rheumatology.
[8] Market Research Future. (2022). "Colchicine Market Analysis." MRFR.