ATAZANAVIR SULFATE Drug Patent Profile
✉ Email this page to a colleague
When do Atazanavir Sulfate patents expire, and what generic alternatives are available?
Atazanavir Sulfate is a drug marketed by Amneal, Aurobindo Pharma, Cipla, Hetero Labs Ltd Iii, Laurus, Mylan, Teva Pharms Usa, and Zydus Pharms. and is included in eight NDAs.
The generic ingredient in ATAZANAVIR SULFATE is atazanavir sulfate. There are twenty-five drug master file entries for this compound. Six suppliers are listed for this compound. Additional details are available on the atazanavir sulfate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Atazanavir Sulfate
A generic version of ATAZANAVIR SULFATE was approved as atazanavir sulfate by TEVA PHARMS USA on April 22nd, 2014.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for ATAZANAVIR SULFATE?
- What are the global sales for ATAZANAVIR SULFATE?
- What is Average Wholesale Price for ATAZANAVIR SULFATE?
Summary for ATAZANAVIR SULFATE
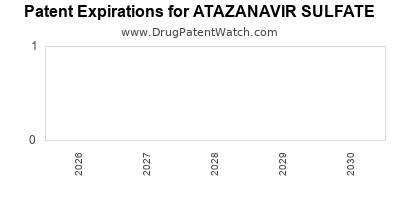
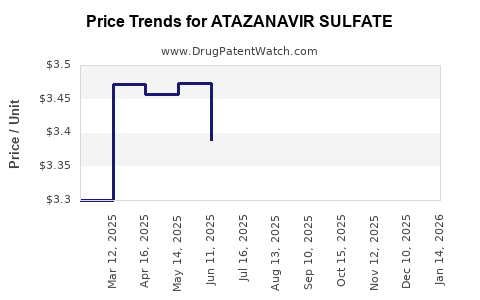
Recent Clinical Trials for ATAZANAVIR SULFATE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| ViiV Healthcare | Phase 4 |
| GlaxoSmithKline | Phase 4 |
Pharmacology for ATAZANAVIR SULFATE
Medical Subject Heading (MeSH) Categories for ATAZANAVIR SULFATE
Anatomical Therapeutic Chemical (ATC) Classes for ATAZANAVIR SULFATE
Paragraph IV (Patent) Challenges for ATAZANAVIR SULFATE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| REYATAZ | Capsules | atazanavir sulfate | 100 mg and 150 mg | 021567 | 1 | 2010-03-19 |
| REYATAZ | Capsules | atazanavir sulfate | 200 mg | 021567 | 1 | 2010-02-16 |
| REYATAZ | Capsules | atazanavir sulfate | 300 mg | 021567 | 1 | 2009-07-20 |
US Patents and Regulatory Information for ATAZANAVIR SULFATE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zydus Pharms | ATAZANAVIR SULFATE | atazanavir sulfate | CAPSULE;ORAL | 210575-003 | Jun 4, 2020 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Amneal | ATAZANAVIR SULFATE | atazanavir sulfate | CAPSULE;ORAL | 209717-001 | Jun 1, 2020 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Laurus | ATAZANAVIR SULFATE | atazanavir sulfate | CAPSULE;ORAL | 212579-003 | Apr 30, 2021 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Teva Pharms Usa | ATAZANAVIR SULFATE | atazanavir sulfate | CAPSULE;ORAL | 091673-003 | Apr 22, 2014 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for ATAZANAVIR SULFATE
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Bristol-Myers Squibb Pharma EEIG | Reyataz | atazanavir sulfate | EMEA/H/C/000494Reyataz capsules, co-administered with low dose ritonavir, are indicated for the treatment of HIV-1 infected adults and paediatric patients 6 years of age and older in combination with other antiretroviral medicinal products (see section 4.2).Based on available virological and clinical data from adult patients, no benefit is expected in patients with strains resistant to multiple protease inhibitors (≥ 4 PI mutations).The choice of Reyataz in treatment experienced adult and paediatric patients should be based on individual viral resistance testing and the patient’s treatment history (see sections 4.4 and 5.1).Reyataz oral powder, co-administered with low dose ritonavir, is indicated in combination with other antiretroviral medicinal products for the treatment of HIV-1 infected paediatric patients at least 3 months of age and weighing at least 5 kg (see section 4.2).Based on available virological and clinical data from adult patients, no benefit is expected in patients with strains resistant to multiple protease inhibitors ( 4 PI mutations). The choice of Reyataz in treatment experienced adult and paediatric patients should be based on individual viral resistance testing and the patient’s treatment history (see sections 4.4 and 5.1). | Authorised | no | no | no | 2004-03-01 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
Market Dynamics and Financial Trajectory for Atazanavir Sulfate
More… ↓
