Last updated: July 27, 2025
Introduction
Amoxicillin, a broad-spectrum beta-lactam antibiotic, remains a cornerstone in antimicrobial therapy globally. Since its introduction in the late 20th century, it has maintained a pivotal role in bacterial infection management, driven by its efficacy, safety profile, and versatility. This analysis delves into the evolving market landscape, including demand trends, competitive forces, regulatory influences, and financial trajectories shaping amoxicillin’s future as a pharmaceutical asset.
Market Landscape and Demand Drivers
Global Prevalence of Bacterial Infections
The persistent high incidence of bacterial infections—respiratory tract infections, urinary tract infections, and skin infections—continues to sustain demand for amoxicillin. The World Health Organization (WHO) estimates that pneumonia and bacterial infections account for over 2 million deaths annually, underscoring the need for accessible and effective antibiotics like amoxicillin[1].
Healthcare Access and Prescription Trends
Increased healthcare accessibility in emerging markets bolsters antibiotic prescriptions. According to IQVIA, amoxicillin remains one of the most dispensed antibiotics worldwide, favored for its broad-spectrum activity and affordability. The shift toward outpatient care further amplifies the volume of prescriptions, given amoxicillin's suitability for oral administration.
Antimicrobial Stewardship and Resistance Concerns
Rising antimicrobial resistance (AMR), especially among common pathogens like Streptococcus pneumoniae and Escherichia coli, poses significant challenges. Resistance to amoxicillin has prompted changes in prescribing protocols, emphasizing combination therapies (e.g., amoxicillin-clavulanate) and stewardship initiatives to preserve its efficacy. While resistance may temper growth somewhat, continuous innovation and policy shifts influence supply and demand dynamics.
Product Availability and Manufacturing Landscape
Established Production and Generic Presence
Amoxicillin is predominantly manufactured by multiple generic pharmaceutical companies. The low-cost, off-patent status facilitates extensive global production, making it a readily available treatment option. The competition drives prices downward, impacting profit margins but ensuring widespread accessibility.
Supply Chain Considerations
Global supply chains, especially for active pharmaceutical ingredients (APIs), impact market stability. Disruptions caused by geopolitical tensions or regulatory restrictions can lead to shortages or price fluctuations. Recent supply chain disturbances due to COVID-19 underscored vulnerabilities in raw material sourcing for antibiotics.
Regulatory and Patent Considerations
Patent Expiry and Market Entry
Amoxicillin’s original patents expired in the early 2000s. Subsequent patent expirations of key formulations opened the market to numerous generic manufacturers, intensifying competition. Regulatory approvals for various formulations and combination products continue to expand market options.
Regulatory Environment and Approvals
Stringent regulations governing antibiotic use, including criteria for approval and quality standards, influence market dynamics. Regulatory agencies, such as the FDA and EMA, enforce antimicrobial stewardship policies, potentially restricting off-label uses and shaping market availability.
Competitive Dynamics and Substitutes
Emergence of Alternatives
While amoxicillin remains dominant, newer antibiotics with enhanced activity and broader spectrum—like cephalosporins and macrolides—offer alternatives. Additionally, increased focus on resistance management leads clinicians to consider other classes, somewhat constraining demand growth.
Innovative Combination Formulations
Development of fixed-dose combinations (e.g., amoxicillin/clavulanic acid) broadens therapeutic applications, addressing resistant strains. Such formulations often command higher prices, improving margins for manufacturers and expanding market segments.
Financial Trajectory and Revenue Projections
Historical Revenue Performance
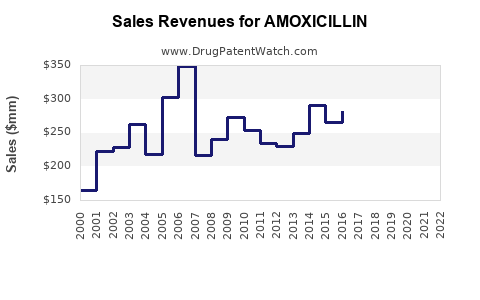
Amoxicillin’s revenue has experienced stable, albeit modest, growth. According to global drug sales data, the antibiotic segment contributed significantly to the revenue streams of major pharmaceutical companies like GlaxoSmithKline and Sandoz. Generic competition, however, has compressed profit margins, necessitating efficiency improvements.
Future Growth Factors
Projected growth will depend on several variables:
- Infection Rates: Continuing high prevalence of bacterial infections sustains baseline demand.
- Antimicrobial Resistance: Resistance patterns may curtail use, though innovation in combination therapies could offset this.
- Regulatory Changes: Adoption or restriction policies impact supply and sales.
- Emergence of Novel Formulations: Extended-release and combination formulations could command premium pricing, influencing revenues.
Market Forecasts
Industry analysts project a compound annual growth rate (CAGR) of approximately 1-3% for the global amoxicillin market over the next five years[2]. The market is expected to stabilize, with growth driven primarily by emerging markets and new formulations, though it faces headwinds from resistance and regulatory constraints.
Geographical Market Trends
North America and Europe
High standards of antimicrobial stewardship and regulatory scrutiny have somewhat constrained volume growth. Nevertheless, high-value formulations and combination products maintain healthy margins.
Asia-Pacific and Latin America
Rapid healthcare infrastructure expansion, increased prescription rates, and high infection burdens drive substantial demand. These regions represent the most significant growth opportunities, especially for generic manufacturers.
Emerging Markets
Government initiatives and efforts to improve access to antibiotics foster increased consumption. However, regulatory challenges and quality concerns could influence market stability.
Impact of COVID-19 Pandemic
The pandemic underscored the critical role of antibiotics in secondary bacterial infections associated with viral illnesses. Initially, disruptions in supply chains hampered production, but the long-term impact includes increased awareness of bacterial infections and potential rise in demand. Conversely, heightened antimicrobial stewardship efforts aimed at curbing overuse may blunt growth.
Regulatory and Ethical Considerations
Increasing global emphasis on combating antimicrobial resistance compels regulatory agencies to tighten approval processes, control distribution, and promote responsible prescribing. These measures influence market accessibility and pricing strategies. Companies integrating stewardship principles into product development may secure favorable regulatory pathways and market positioning.
Conclusion
The market dynamics for amoxicillin remain shaped by persistent demand driven by global infection rates, widespread generic production, and evolving resistance patterns. While the overall financial trajectory suggests modest growth, opportunities exist in developing advanced formulations, expanding into emerging markets, and optimizing supply chains. Carefully navigating regulatory landscapes and resistance challenges is vital for sustaining profitability and market relevance.
Key Takeaways
- Stable Demand with Growth Potential: Amoxicillin’s central role in infection management ensures baseline demand, especially in emerging markets experiencing healthcare expansion.
- Pricing and Competition: Extensive generic availability exerts downward pressure on prices; innovation in formulations offers avenues for higher margins.
- Resistance Challenges: Growing antimicrobial resistance necessitates strategic stewardship and product reformulation, influencing market offerings.
- Regulatory Environment: Tightening policies around antibiotic use impact market access, requiring adaptive strategies from manufacturers.
- Emerging Markets as Growth Engines: Asia-Pacific and Latin America present significant opportunities due to increased infection burdens and healthcare infrastructure improvements.
FAQs
-
What factors influence the price dynamics of amoxicillin?
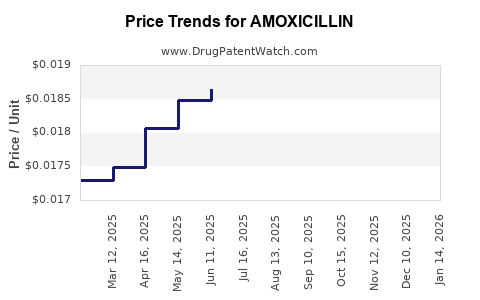
Price fluctuations are primarily driven by generic competition, supply chain stability, regulatory policies, and the emergence of resistance. Extensive manufacturing of generics keeps prices low, while supply disruptions or patent protections in certain formulations can temporarily impact pricing.
-
How does antimicrobial resistance impact the future of amoxicillin?
Rising resistance reduces the drug’s efficacy against certain pathogens, leading to decreased usage or the need for combination therapies. This trend compels pharmaceutical companies to innovate or reformulate to maintain market relevance.
-
Are there new formulations of amoxicillin that could influence its market share?
Yes. Extended-release forms, fixed-dose combinations, and formulations targeting resistant strains can command premium pricing and expand therapeutic uses, influencing future market share positively.
-
What role do regulatory agencies play in the amoxicillin market?
They oversee approval standards, quality control, and prescribing guidelines. Stricter regulations aim to curb misuse, influence formulation approvals, and guide stewardship efforts, thereby shaping demand and supply.
-
What are the key markets to watch for amoxicillin growth?
Emerging markets in Asia-Pacific and Latin America are pivotal, given their expanding healthcare systems and infection burdens. Developed regions focus on optimized use and stewardship, which impacts volume and value growth.
References
[1] World Health Organization. (2021). Global report on antimicrobial resistance.
[2] MarketWatch. (2022). Amoxicillin market forecasts and trends.